Blood Res.
2016 Jun;51(2):95-101. 10.5045/br.2016.51.2.95.
Platelet count recovery after intravenous immunoglobulin predicts a favorable outcome in children with immune thrombocytopenia
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea. choihs1786@snubh.org
- KMID: 2309210
- DOI: http://doi.org/10.5045/br.2016.51.2.95
Abstract
- BACKGROUND
Childhood immune thrombocytopenic purpura (ITP) is a common acquired bleeding disorder. Even though most children recover, either spontaneously or with therapy, 10-20% of newly diagnosed ITP cases have a chronic course beyond 12 months. This study evaluated whether clinical and laboratory findings can predict the response to intravenous immunoglobulin (IVIG) and progression to persistent or chronic ITP in children.
METHODS
During the period between March 2003 and June 2015, we retrospectively analyzed 72 children, newly diagnosed with ITP, who received IVIG treatment. Peripheral blood counts were obtained at diagnosis and at 1, 3, 6, and 12 months after IVIG treatment.
RESULTS
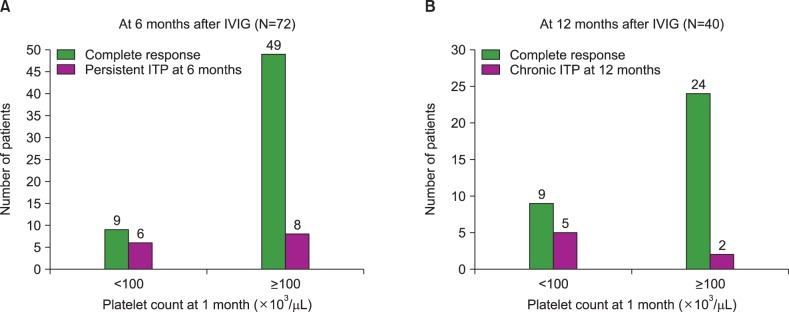
After 6 months of IVIG treatment, 14 of 72 patients (19.4%) had persistent ITP, and after 12 months, 7 of 40 patients (17.5%) had chronic ITP. Age at diagnosis, gender, history of viral infection, or vaccination before disease onset were not statistically correlated with platelet recovery at 6 and 12 months. However, a platelet count recovery of ≥100×10(3)/µL at 1 and 3 months was significantly correlated with platelet recovery at 6 (P<0.001 and P<0.001, respectively) and 12 (P=0.007 and P=0.004, respectively) months.
CONCLUSION
This study demonstrated that early platelet count recovery, at 1 and 3 months after IVIG treatment, predicts a short disease duration and a favorable outcome in children with newly diagnosed ITP. Further investigation in a larger group of patients is warranted to validate these findings.
MeSH Terms
Figure
Cited by 1 articles
-
Management of immune thrombocytopenia: Korean experts recommendation in 2017
Jun Ho Jang, Ji Yoon Kim, Yeung-Chul Mun, Soo-Mee Bang, Yeon Jung Lim, Dong-Yeop Shin, Young Bae Choi, Ho-Young Yhim, Jong Wook Lee, Hoon Kook,
Blood Res. 2017;52(4):254-263. doi: 10.5045/br.2017.52.4.254.
Reference
-
1. Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011; 117:4190–4207. PMID: 21325604.
Article2. Neunert CE. Current management of immune thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2013; 2013:276–282. PMID: 24319191.
Article3. Stasi R, Newland AC. ITP: a historical perspective. Br J Haematol. 2011; 153:437–450. PMID: 21466538.
Article4. Labarque V, Van Geet C. Clinical practice: immune thrombocytopenia in paediatrics. Eur J Pediatr. 2014; 173:163–172. PMID: 24390128.
Article5. Schultz CL, Mitra N, Schapira MM, Lambert MP. Influence of the American Society of Hematology guidelines on the management of newly diagnosed childhood immune thrombocytopenia. JAMA Pediatr. 2014; 168:e142214. PMID: 25288142.
Article6. Neunert CE, Buchanan GR, Imbach P, et al. Severe hemorrhage in children with newly diagnosed immune thrombocytopenic purpura. Blood. 2008; 112:4003–4008. PMID: 18698007.
Article7. Imbach P, Barandun S, d'Apuzzo V, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981; 1:1228–1231. PMID: 6112565.
Article8. Bussel JB, Hilgartner MW. The use and mechanism of action of intravenous immunoglobulin in the treatment of immune haematologic disease. Br J Haematol. 1984; 56:1–7. PMID: 6367804.
Article9. Beck CE, Nathan PC, Parkin PC, Blanchette VS, Macarthur C. Corticosteroids versus intravenous immune globulin for the treatment of acute immune thrombocytopenic purpura in children: a systematic review and meta-analysis of randomized controlled trials. J Pediatr. 2005; 147:521–527. PMID: 16227040.
Article10. Rosthøj S, Hedlund-Treutiger I, Rajantie J, et al. Duration and morbidity of newly diagnosed idiopathic thrombocytopenic purpura in children: A prospective Nordic study of an unselected cohort. J Pediatr. 2003; 143:302–307. PMID: 14517509.
Article11. Yacobovich J, Revel-Vilk S, Tamary H. Childhood immune thrombocytopenia--who will spontaneously recover? Semin Hematol. 2013; 50(Suppl 1):S71–S74. PMID: 23664522.
Article12. Heitink-Pollé KM, Nijsten J, Boonacker CW, de Haas M, Bruin MC. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: a systematic review and meta-analysis. Blood. 2014; 124:3295–3307. PMID: 25305206.
Article13. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009; 113:2386–2393. PMID: 19005182.
Article14. Zeller B, Rajantie J, Hedlund-Treutiger I, et al. Childhood idiopathic thrombocytopenic purpura in the Nordic countries: epidemiology and predictors of chronic disease. Acta Paediatr. 2005; 94:178–184. PMID: 15981751.
Article15. Donato H, Picón A, Martinez M, et al. Demographic data, natural history, and prognostic factors of idiopathic thrombocytopenic purpura in children: a multicentered study from Argentina. Pediatr Blood Cancer. 2009; 52:491–496. PMID: 19058214.
Article16. Ahmed I, Rajpurkar M, Thomas R, Chitlur M. Initial lymphocyte count and the development of persistent/chronic immune thrombocytopenic purpura. Pediatr Blood Cancer. 2010; 55:508–511. PMID: 20658623.
Article17. Ho WL, Lee CC, Chen CJ, et al. Clinical features, prognostic factors, and their relationship with antiplatelet antibodies in children with immune thrombocytopenia. J Pediatr Hematol Oncol. 2012; 34:6–12. PMID: 22215094.
Article18. Kubota M, Adachi S, Usami I, et al. Characterization of chronic idiopathic thrombocytopenic purpura in Japanese children: a retrospective multi-center study. Int J Hematol. 2010; 91:252–257. PMID: 20049564.
Article19. Deel MD, Kong M, Cross KP, Bertolone SJ. Absolute lymphocyte counts as prognostic indicators for immune thrombocytopenia outcomes in children. Pediatr Blood Cancer. 2013; 60:1967–1974. PMID: 24038723.
Article20. Revel-Vilk S, Yacobovich J, Frank S, et al. Age and duration of bleeding symptoms at diagnosis best predict resolution of childhood immune thrombocytopenia at 3, 6, and 12 months. J Pediatr. 2013; 163:1335–1339. PMID: 23891349.
Article21. Evim MS, Baytan B, Güneş AM. Childhood immune thrombocytopenia: long-term follow-up data evaluated by the criteria of the international working group on immune thrombocytopenic purpura. Turk J Haematol. 2014; 31:32–39. PMID: 24764727.
Article22. Yildiz I, Ozdemir N, Celkan T, et al. Initial management of childhood acute immune thrombocytopenia: single-center experience of 32 years. Pediatr Hematol Oncol. 2015; 32:406–414. PMID: 26154620.
Article23. Tamminga R, Berchtold W, Bruin M, Buchanan GR, Kühne T. Possible lower rate of chronic ITP after IVIG for acute childhood ITP an analysis from registry I of the Intercontinental Cooperative ITP Study Group (ICIS). Br J Haematol. 2009; 146:180–184. PMID: 19466971.
Article24. Morimoto Y, Yoshida N, Kawashima N, Matsumoto K, Kato K. Identification of predictive factors for response to intravenous immunoglobulin treatment in children with immune thrombocytopenia. Int J Hematol. 2014; 99:597–602. PMID: 24573984.
Article25. Kim UH, Lee SI, Lee KS. Early prediction of chronic childhood immune thrombocytopenic purpura according to the response of immunoglobulin treatment. Clin Pediatr Hematol Oncol. 2013; 20:79–85.26. Park SJ, Baek HJ, Lee SU, et al. Helicobacter pylori infection in children with chronic idiopathic thrombocytopenic purpura. Clin Pediatr Hematol Oncol. 2008; 15:83–91.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical course and prognostic factors of childhood immune thrombocytopenia: single center experience of 10 years
- Naproxen-induced Immune Thrombocytopenia: A case report
- A Case of Immune Thrombocytopenic Purpura Accompanying Ulcerative Colitis
- Delayed treatment-free response after romiplostim discontinuation in pediatric chronic immune thrombocytopenia
- Immune thrombocytopenic purpura(ITP)