Lab Anim Res.
2016 Jun;32(2):105-115. 10.5625/lar.2016.32.2.105.
Beneficial effect of diosgenin as a stimulator of NGF on the brain with neuronal damage induced by Aβ-42 accumulation and neurotoxicant injection
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources and Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Biologics Division, Ministry of Food and Drug Administration (MFDS), Cheongju, Korea. cjbae76@gmail.com
- KMID: 2309084
- DOI: http://doi.org/10.5625/lar.2016.32.2.105
Abstract
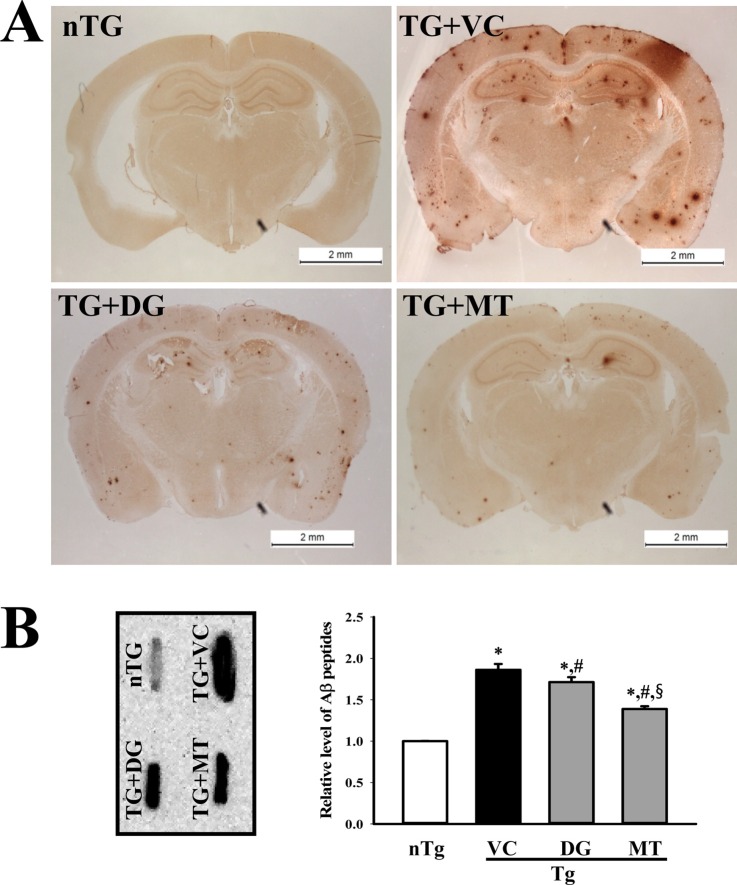
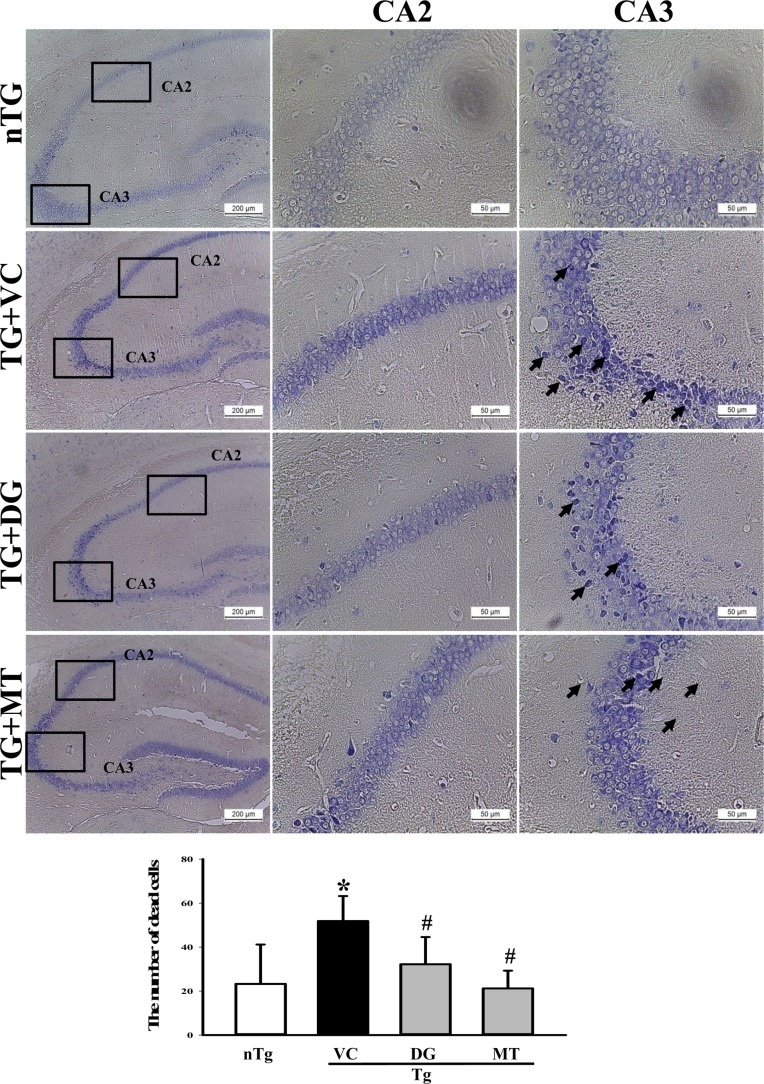
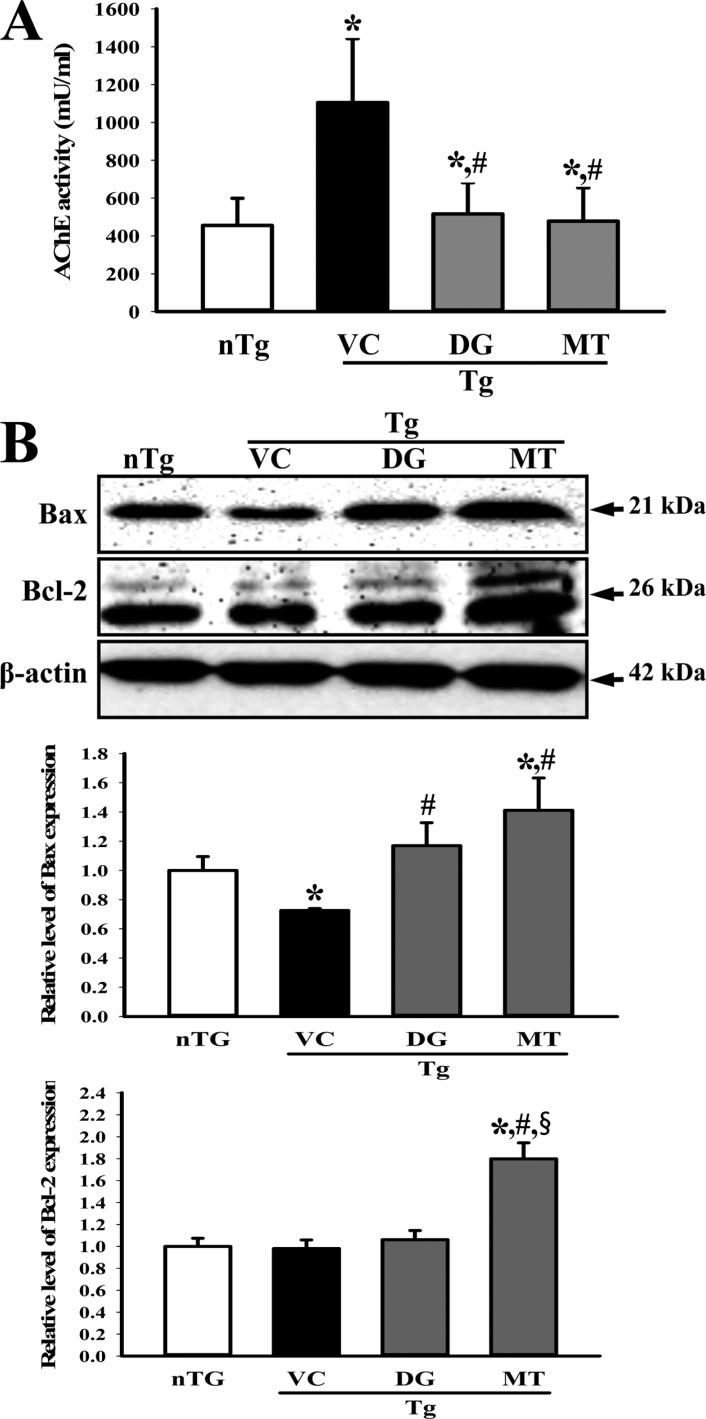
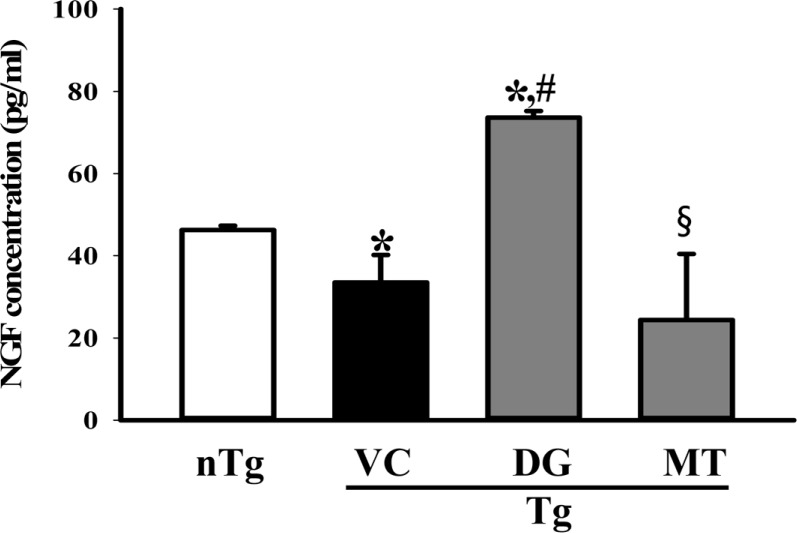
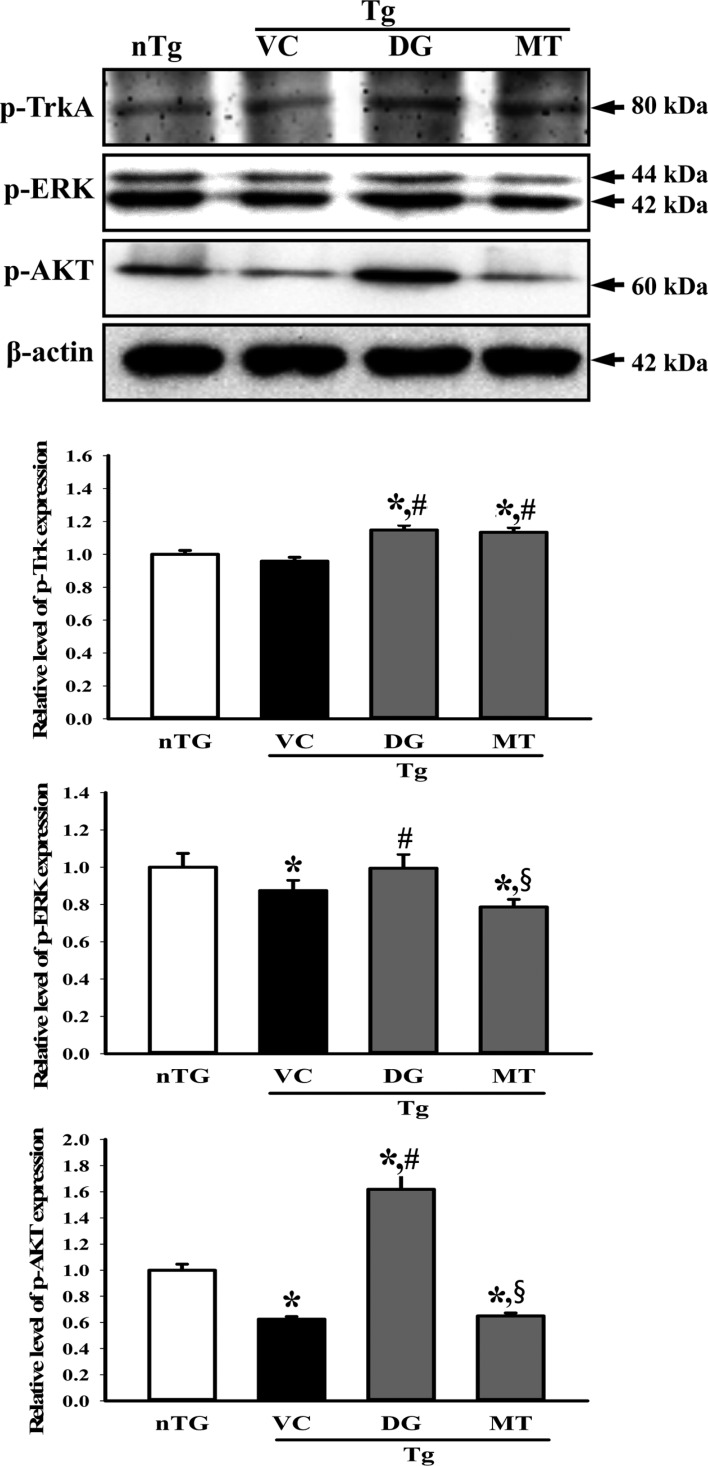
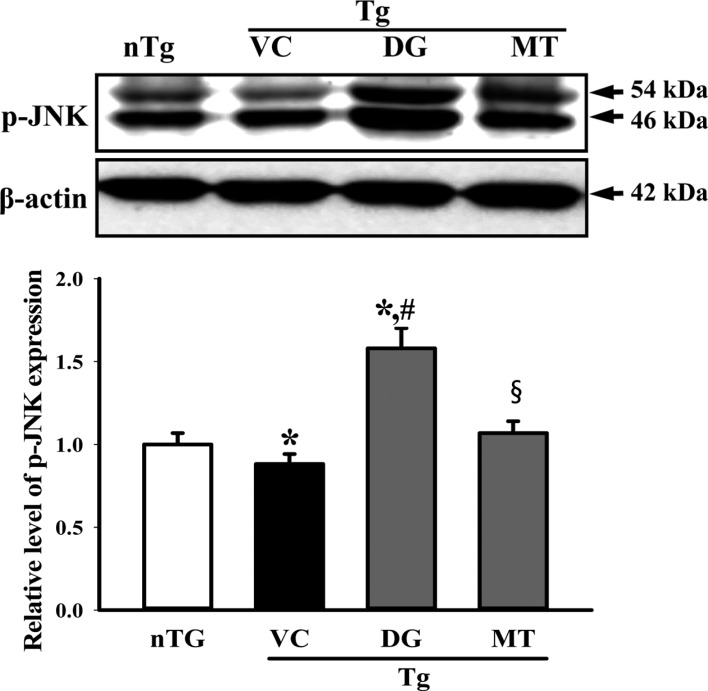
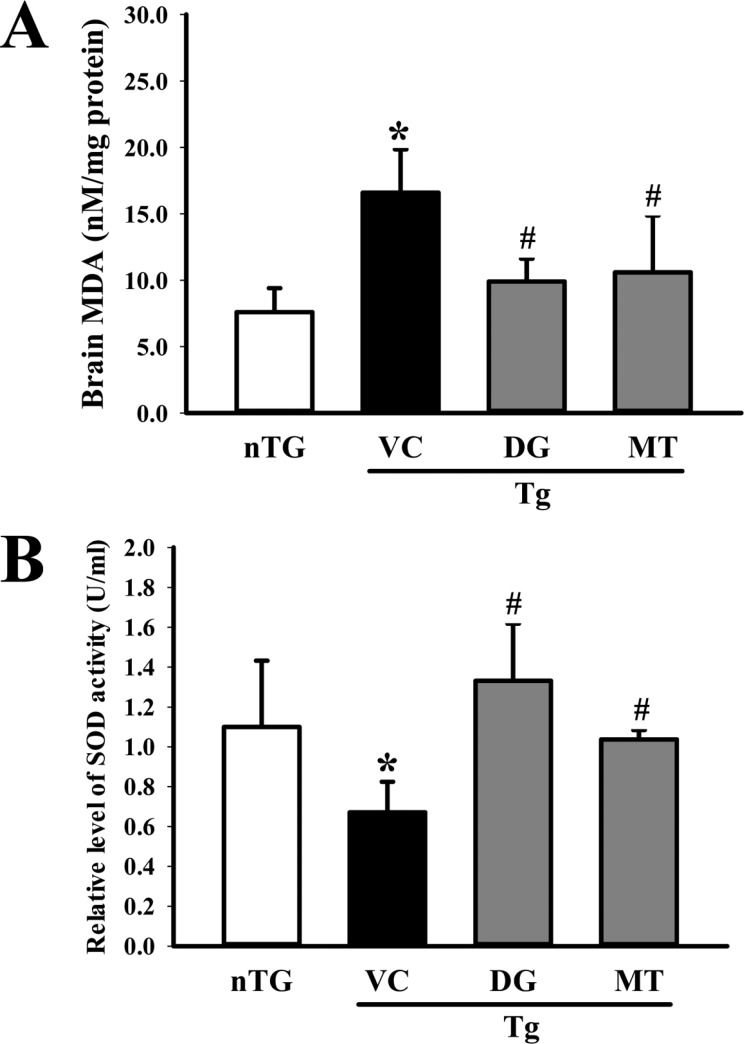
- To investigate the beneficial effects of diosgenin (DG) on the multiple types of brain damage induced by Aβ-42 peptides and neurotoxicants, alterations in the specific aspects of brain functions were measured in trimethyltin (TMT)-injected transgenic 2576 (TG) mice that had been pretreated with DG for 21 days. Multiple types of damage were successfully induced by Aβ-42 accumulation and TMT injection into the brains of TG mice. However, DG treatment significantly reduced the number of Aβ-stained plaques and dead cells in the granule cells layer of the dentate gyrus. Significant suppression of acetylcholinesterase (AChE) activity and Bax/Bcl-2 expression was also observed in the DG treated TG mice (TG+DG group) when compared with those of the vehicle (VC) treated TG mice (TG+VC group). Additionally, the concentration of nerve growth factor (NGF) was dramatically enhanced in TG+DG group, although it was lower in the TG+VC group than the non-transgenic (nTG) group. Furthermore, the decreased phosphorylation of downstream members in the TrkA high affinity receptor signaling pathway in the TG+VC group was significantly recovered in the TG+DG group. A similar pattern was observed in p75NTR expression and JNK phosphorylation in the NGF low affinity receptor signaling pathway. Moreover, superoxide dismutase (SOD) activity was enhanced in the TG+DG group, while the level of malondialdehyde (MDA), a marker of lipid peroxidation, was lower in the TG+DG group than the TG+VC group. These results suggest that DG could exert a wide range of beneficial activities for multiple types of brain damage through stimulation of NGF biosynthesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Ziegler-Graham K, Brookmeyer R, Johnson E, Arrighi HM. Worldwide variation in the doubling time of Alzheimer's disease incidence rates. Alzheimers Dement. 2008; 4(5):316–323. PMID: 18790458.
Article2. Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004; 430(7000):631–639. PMID: 15295589.
Article3. Salloway S, Correia S. Alzheimer disease: time to improve its diagnosis and treatment. Cleve Clin J Med. 2009; 76(1):49–58. PMID: 19122111.
Article4. Henley DB, May PC, Dean RA, Siemers ER. Development of semagacestat (LY450139), a functional gamma-secretase inhibitor, for the treatment of Alzheimer's disease. Expert Opin Pharmacother. 2009; 10(10):1657–1664. PMID: 19527190.5. Dickson TC, Vickers JC. The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer's disease. Neuroscience. 2001; 105(1):99–107. PMID: 11483304.6. Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991; 30(4):572–580. PMID: 1789684.
Article7. Tohda C, Kuboyama T, Komatsu K. Search for natural products related to regeneration of the neuronal network. Neurosignals. 2005; 14(1-2):34–45. PMID: 15956813.
Article8. Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, Mulnard R, Barakos J, Gregg KM, Liu E, Lieberburg I, Schenk D, Black R, Grundman M. Bapineuzumab 201 Clinical Trial Investigators. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009; 73(24):2061–2070. PMID: 19923550.
Article9. Tohda C, Urano T, Umezaki M, Nemere I, Kuboyama T. Diosgenin is an exogenous activator of 1,25D3-MARRS/Pdia3/ERp57 and improves Alzheimers disease pathologies in 5XFAD mice. Sci Rep. 2012; 2:535. PMID: 22837815.
Article10. Baulieu EE, Robel P, Schumacher M. Neurosteroids: beginning of the story. Int Rev Neurobiol. 2001; 46:1–32. PMID: 11599297.
Article11. Papadopoulos V, Lecanu L. Caprospinol: discovery of a steroid drug candidate to treat Alzheimer's disease based on 22R-hydroxycholesterol structure and properties. J Neuroendocrinol. 2012; 24(1):93–101. PMID: 21623958.
Article12. Burkill IH. The organography and the evolution of Dioscoreaceae, the family of the Yams. J Linn Soc (Bot). 1960; 56(367):319–412.
Article13. Yan LL, Zhang YJ, Gao WY, Man SL, Wang Y. In vitro and in vivo anticancer activity of steroid saponins of Paris polyphylla var. yunnanensis. Exp Oncol. 2009; 31(1):27–32. PMID: 19300413.14. Huang CH, Ku CY, Jan TR. Diosgenin attenuates allergen-induced intestinal inflammation and IgE production in a murine model of food allergy. Planta Med. 2009; 75(12):1300–1305. PMID: 19343624.
Article15. Chiu CS, Chiu YJ, Wu LY, Lu TC, Huang TH, Hsieh MT, Lu CY, Peng WH. Diosgenin ameliorates cognition deficit and attenuates oxidative damage in senescent mice induced by D-galactose. Am J Chin Med. 2011; 39(3):551–563. PMID: 21598421.
Article16. Kang TH, Moon E, Hong BN, Choi SZ, Son M, Park JH, Kim SY. Diosgenin from Dioscorea nipponica ameliorates diabetic neuropathy by inducing nerve growth factor. Biol Pharm Bull. 2011; 34(9):1493–1498. PMID: 21881239.17. Tohda C, Lee YA, Goto Y, Nemere I. Diosgenin-induced cognitive enhancement in normal mice is mediated by 1,25D3-MARRS. Sci Rep. 2013; 3:3395. PMID: 24292207.
Article18. Prajapati KD, Sharma SS, Roy N. Upregulation of albumin expression in focal ischemic rat brain. Brain Res. 2010; 1327:118–124. PMID: 20193666.
Article19. Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001; 21(2):372–381. PMID: 11160418.20. Massoulié J, Pezzementi L, Bon S, Krejci E, Vallette FM. Molecular and cellular biology of cholinesterases. Prog Neurobiol. 1993; 41(1):31–91. PMID: 8321908.
Article21. Obara Y, Nakahata N. The signaling pathway of neurotrophic factor biosynthesis. Drug News Perspect. 2002; 15(5):290–298. PMID: 12677225.
Article22. Takei N, Tsukui H, Hatanaka H. Intracellular storage and evoked release of acetylcholine from postnatal rat basal forebrain cholinergic neurons in culture with nerve growth factor. J Neurochem. 1989; 53(5):1405–1410. PMID: 2795008.
Article23. Wyman T, Rohrer D, Kirigiti P, Nichols H, Pilcher K, Nilaver G, Machida C. Promoter-activated expression of nerve growth factor for treatment of neurodegenerative diseases. Gene Ther. 1999; 6(10):1648–1660. PMID: 10516713.
Article24. Furukawa Y, Furukawa S, Satoyoshi E, Hayashi K. Catecholamines induce an increase in nerve growth factor content in the medium of mouse L-M cells. J Biol Chem. 1986; 261(13):6039–6047. PMID: 3700383.
Article25. Furukawa Y, Furukawa S, Ikeda F, Satoyoshi E, Hayashi K. Aliphatic side chain of catecholamine potentiates the stimulatory effect of the catechol part on the synthesis of nerve growth factor. FEBS Lett. 1986; 208(2):258–262. PMID: 3780966.
Article26. Obara Y, Nakahata N, Kita T, Takaya Y, Kobayashi H, Hosoi S, Kiuchi F, Ohta T, Oshima Y, Ohizumi Y. Stimulation of neurotrophic factor secretion from 1321N1 human astrocytoma cells by novel diterpenoids, scabronines A and G. Eur J Pharmacol. 1999; 370(1):79–84. PMID: 10323283.
Article27. Marcotullio MC, Pagiotti R, Maltese F, Oball-Mond Mwankie GN, Hoshino T, Obara Y, Nakahata N. Cyathane diterpenes from Sarcodon cyrneus and evaluation of their activities of neuritegenesis and nerve growth factor production. Bioorg Med Chem. 2007; 15(8):2878–2882. PMID: 17320402.
Article28. Ma BJ, Shen JW, Yu HY, Ruan Y, Wu TT, Zhao X. Hericenones and erinacines: stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycol. 2010; 1(2):92–98.29. Kumar S, Walter J. Phosphorylation of amyloid beta (Aβ) peptides - a trigger for formation of toxic aggregates in Alzheimer's disease. Aging (Albany NY). 2011; 3(8):803–812. PMID: 21869458.
Article30. Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012; 148(6):1204–1222. PMID: 22424230.
Article31. Leuner K, Schutt T, Kurz C, Eckert SH, Schiller C, Occhipinti A, Mai S, Jendrach M, Eckert GP, Kruse SE, Palmiter RD, Brandt U, Drose S, Wittig I, Willem M, Haass C, Reichert AS, Muller WE. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid Redox Signal. 2012; 16(12):1421–1433. PMID: 22229260.
Article32. Leuner K, Muller WE, Reichert AS. From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer's disease. Mol Neurobiol. 2012; 46(1):186–193. PMID: 22833458.
Article33. Park D, Joo SS, Kim TK, Lee SH, Kang H, Lee HJ, Lim I, Matsuo A, Tooyama I, Kim YB, Kim SU. Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant. 2012; 21(1):365–371. PMID: 21929870.
Article34. Nunes-Tavares N, Santos LE, Stutz B, Brito-Moreira J, Klein WL, Ferreira ST, de Mello FG. Inhibition of choline acetyltransferase as a mechanism for cholinergic dysfunction induced by amyloid-β peptide oligomers. J Biol Chem. 2012; 287(23):19377–19385. PMID: 22505713.
Article35. Nabeshima T, Noda Y, Kamei H. Anti-dementia drugs for Alzheimer disease in present and future. Nihon Yakurigaku Zasshi. 2002; 120(1):24–29.36. Kar S, Slowikowski SP, Westaway D, Mount HT. Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer's disease. J Psychiatry Neurosci. 2004; 29(6):427–441. PMID: 15644984.37. Ingkaninan K, Temkitthawon P, Chuenchom K, Yuyaem T, Thongnoi W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J Ethnopharmacol. 2003; 89(2-3):261–264. PMID: 14611889.
Article38. Chattipakorn S, Pongpanparadorn A, Pratchayasakul W, Pongchaidacha A, Ingkaninan K, Chattipakorn N. Tabernaemontana divaricata extract inhibits neuronal acetylcholinesterase activity in rats. J Ethnopharmacol. 2007; 110(1):61–68. PMID: 17023131.39. Nakdook W, Khongsombat O, Taepavarapruk P, Taepavarapruk N, Ingkaninan K. The effects of Tabernaemontana divaricata root extract on amyloid beta-peptide 25-35 peptides induced cognitive deficits in mice. J Ethnopharmacol. 2010; 130(1):122–126. PMID: 20435125.40. Nitta A, Ogihara Y, Onishi J, Hasegawa T, Furukawa S, Nabeshima T. Oral administration of propentofylline, a stimulator of nerve growth factor (NGF) synthesis, recovers cholinergic neuronal dysfunction induced by the infusion of anti-NGF antibody into the rat septum. Behav Brain Res. 1997; 83(1-2):201–204. PMID: 9062684.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory effect of the leaves and stems of Actinidia arguta on Aβ (25–35)-induced neuronal cell death and memory impairment
- Long Noncoding RNA NEAT1 Aggravates Aβ-Induced Neuronal Damage by Targeting miR-107 in Alzheimer's Disease
- Protective effect of the aerial parts of Silybum marianum against amyloid β protein (25-35)-induced neuronal death in cultured neurons
- Beta-amyloid imaging in dementia
- The Novel Implication of Androgen in Diabetes-induced Alzheimer's Disease