Kosin Med J.
2014 Dec;29(2):135-140. 10.7180/kmj.2014.29.2.135.
Nerve-Sparing Cryoablation for the Treatment of Primary Prostate Cancer: the Preliminary Report
- Affiliations
-
- 1Deparment of Urology, College of Medicine, Kosin University, Busan, Korea.
- KMID: 2308498
- DOI: http://doi.org/10.7180/kmj.2014.29.2.135
Abstract
- BACKGROUND
To present a pilot study of nerve-sparing cryoablation for the treatment of primary prostate cancer.
MATERIALS AND METHODS
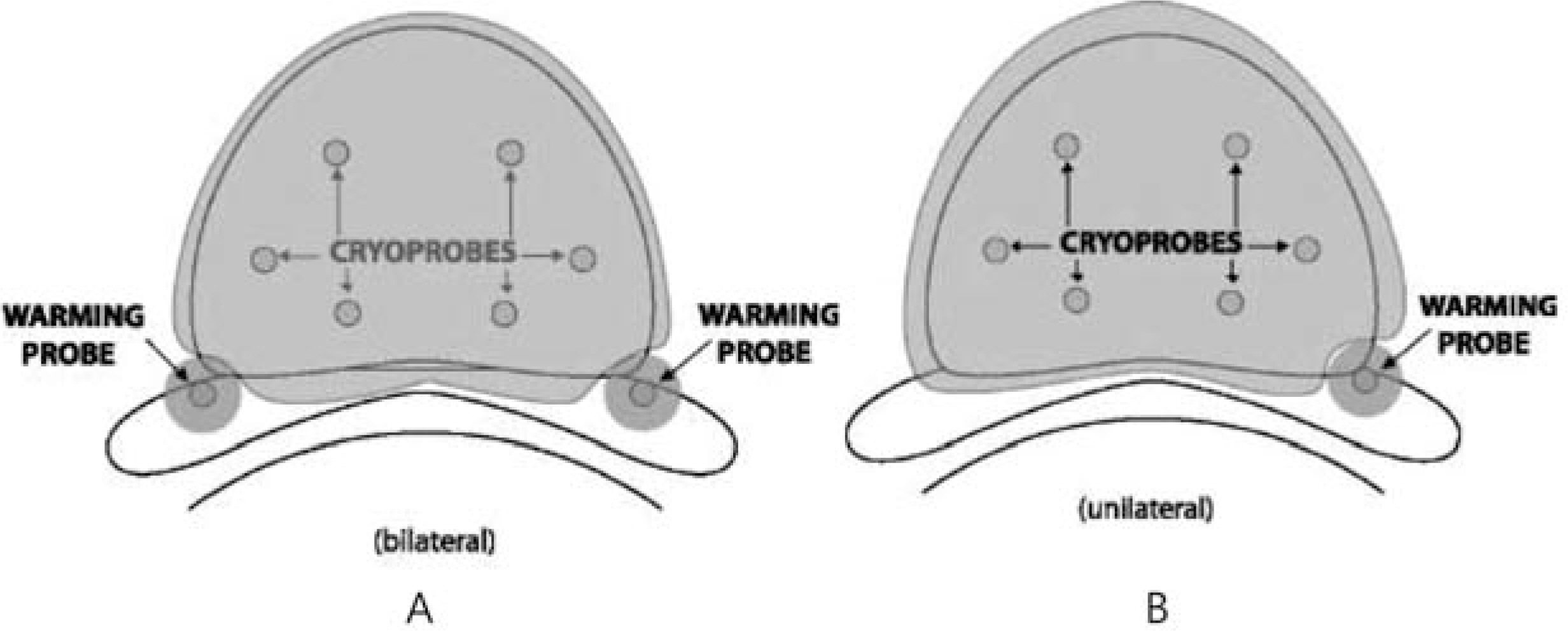
Between 2008 and 2011, 9 patients underwent nerve-sparing cryoablation (unilateral 5, bilateral 4 patients). One neurovascular bundle (NVB) was spared on the side opposite the positive biopsy, and two NVBs were spared when indicated and possible. Just before the start of freezing, a 22-gauge spinal needle was placed into Denonvilliers fascia using a transperineal route, and normal saline was injected to separate the rectum from the prostate. The prostate-specific antigen (PSA) level was sampled every 3 months for the first 2 years and then every 6 months thereafter. Patients were considered to have a stable PSA if they had two consecutive PSA measurements without a rise.
RESULTS
The follow-up was 40-months (19-66 months). All patients had stable PSA levels at last follow-up. Potency (defined as an erection sufficient to complete intercourse to the satisfaction of the patient) was maintained in 4 of 9 patients, 5 were potent with phosphodiesterase 5 inhibitors or intracavernosal injection.
CONCLUSIONS
Nerve-sparing cryoablation, in which one or two neurovascular bundle is spared, showed the possibility of preserving potency in most patients without compromising cancer control. These preliminary results warrant further study.
Keyword
MeSH Terms
Figure
Reference
-
1.Walsh PC., and Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982. 128:492–5.
Article2.Talcott JA., Reiker P., Propert KJ., Clark JA., Wishnow KI., Loughlin KR, et al. Patient reported impotence and incontinence after nerve-sparing radical prostatectomy. J Natl Cancer Inst. 1989. 16:1117–23.3.Ficarra V., Novara G., Ahlering TE., Costello A., Eastham JA., Graefen M, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012. 62:418–30.
Article4.Wahle S., Reznicek M., Fallon B., Platz C., Williams R. Incidence of surgical margin involvement in various forms of radical prostatectomy. Urology. 1990. 36:23–6.
Article5.Vaidya A., Hawke C., Tiguert R., Civantos F., Soloway M. Intraoperative T staging in radical retropubic prostatectomy: is it reliable? Urology. 2001. 57:949–54.
Article6.Bonney WW., Fallon B., Gerber WL., Hawtrey CE., Loening SA., Narayana AS, et al. Cryosurgery in prostatic cancer survival. Urology. 1982. 14:37–42.
Article7.Onik GM., Cohen K., Reyes GD., Rubinsky B., Chang Z: Baust J. Transrectal ultrasound-guided percutaneous radical cryosurgical ablation of the prostate. Cancer. 1993. 72:1291–9.
Article8.Wong WS., Chinn DO., Chinn M., Chinn J., Tom WL., Tom WL. Cryosurgery as a treatment for prostate carcinoma: results and complications. Cancer. 1997. 79:963–74.9.Robinson JW., Donnelly BJ., Saliken JC., Weber BA., Ernst S., Rewcastle JC. Quality of life and sexuality of men with prostate cancer 3 years after cryosurgery. Urology. 2002. 60:12–8.
Article10.Onik G., Narayan P., Vaughan D., Dineen M., Brunelle D. Focal “nerve-sparing” cryosurgery for treatment of primary prostate cancer: a new approach to preserving potency. Urology. 2002. 60:109–14.
Article11.Roach M 3rd., Hanks G., Thames H Jr., Schellhammer P., Shipley WU., Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Concensus Conference. Int J Radiat Oncol Biol Phys. 2006. 65:965–74.12.Villers A., McNeal JE., Freiha FS., Stamey TA. Multiple cancers in the prostate: morphologic features of clinically recognized vs. incidental tumors. Cancer. 1992. 70:2312–38.13.Cookson MS. Update on transrectal ultrasound guided needle biopsy of the prostate. Mol Urol. 2000. 4:93–7.14.Sanchez-Ortiz RF., Broderick GA., Rovner ES., Wein AJ., Whittington R. Malkowicz SB. Erectile function and quality of life after interstitial radiation therapy for prostate cancer. Int J Impot Res. 2000. 12:SI8–24.
Article15.Zelefsky MJ., Hollister T., Raben A., Matthews S., Wallner KE. Five year biochemical outcome and toxicity with transperineal CT-planned permanent 1-125 prostate implantation for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2000. 47:1261–6.16.O'Sullivan DC., Barrett DM., Colby TV., Lieber MM., Cupps RE. Effect of external beam radiation therapy on prostatic carcinoma DNA content as measured by static image cytometry. Eur Urol. 1992. 21:235–59.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nerve-sparing techniques and results in robot-assisted radical prostatectomy
- The Initial Experience with 3rd Generation Nephron-sparing Cryoablation for Renal Tumor
- Inducing of Complete Necrosis of Recurred Lung Cancer by Cryoablation: A Case Report
- A Prospective Pilot Study Investigating Performance of 18F‑Fluciclovine PET Imaging for Detection of Prostate Cancer 2 Years Following Primary Partial Gland Cryoablation
- Pain during Transrectal Ultrasound-Guided Prostate Biopsy and the Role of Periprostatic Nerve Block: What Radiologists Should Know