Korean J Hematol.
2006 Dec;41(4):235-242. 10.5045/kjh.2006.41.4.235.
Current Status of Hematopoietic Stem Cell Transplantation in Korean Children
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, The Catholic University of Korea, Korea.
- 2Department of Pediatrics, College of Medicine, Seoul National University, Korea.
- 3Department of Pediatrics, College of Medicine, Sungkyunkwan University, Korea.
- 4Department of Pediatrics, College of Medicine, Chonnam National University, Korea. tjhwang@chonnam.ac.kr
- 5Department of Pediatrics, College of Medicine, Inha University, Korea.
- 6Department of Pediatrics, College of Medicine, National Cancer Center, Korea.
- 7Department of Pediatrics, College of Medicine, Yonsei University Wonju College of Medicine, Korea.
- 8Department of Pediatrics, College of Medicine, Ulsan University, Korea.
- 9Department of Pediatrics, College of Medicine, Ewha Women's University, Korea.
- 10Department of Pediatrics, College of Medicine, Yonsei University, Korea.
- 11Department of Pediatrics, College of Medicine, Korea University, Korea.
- 12Department of Pediatrics, College of Medicine, Inje University, Korea.
- 13Department of Pediatrics, College of Medicine, Dong-A University, Korea.
- 14Department of Pediatrics, College of Medicine, Pusan National University, Korea.
- 15Department of Pediatrics, College of Medicine, Gyeongsang National University, Korea.
- 16Department of Pediatrics, Gachon University of Medicine and Science, Korea.
- 17Department of Pediatrics, College of Medicine, Yeungnam University, Korea.
- 18Department of Pediatrics, College of Medicine, Chonbuk National University, Korea.
- KMID: 2305209
- DOI: http://doi.org/10.5045/kjh.2006.41.4.235
Abstract
-
BACKGROUND: Hematopoietic stem cell transplantation (HSCT) is one of the most important armamentarium against various hematologic malignancies or some solid tumors. We investigated the number of patients who might need transplants and compared with that of actual transplants to conceptualize current status and circumstances of HSCTs in Korean children.
METHODS
Questionnaires were sent to Korean Society of Hematopoietic Stem Cell Transplantation (KSHSCT) members who were taking care of children with malignancies or hematologic diseases. Almost all of the newly diagnosed patients between Jan, 1st and Dec, 31st, 2003 were enrolled in the study.
RESULTS
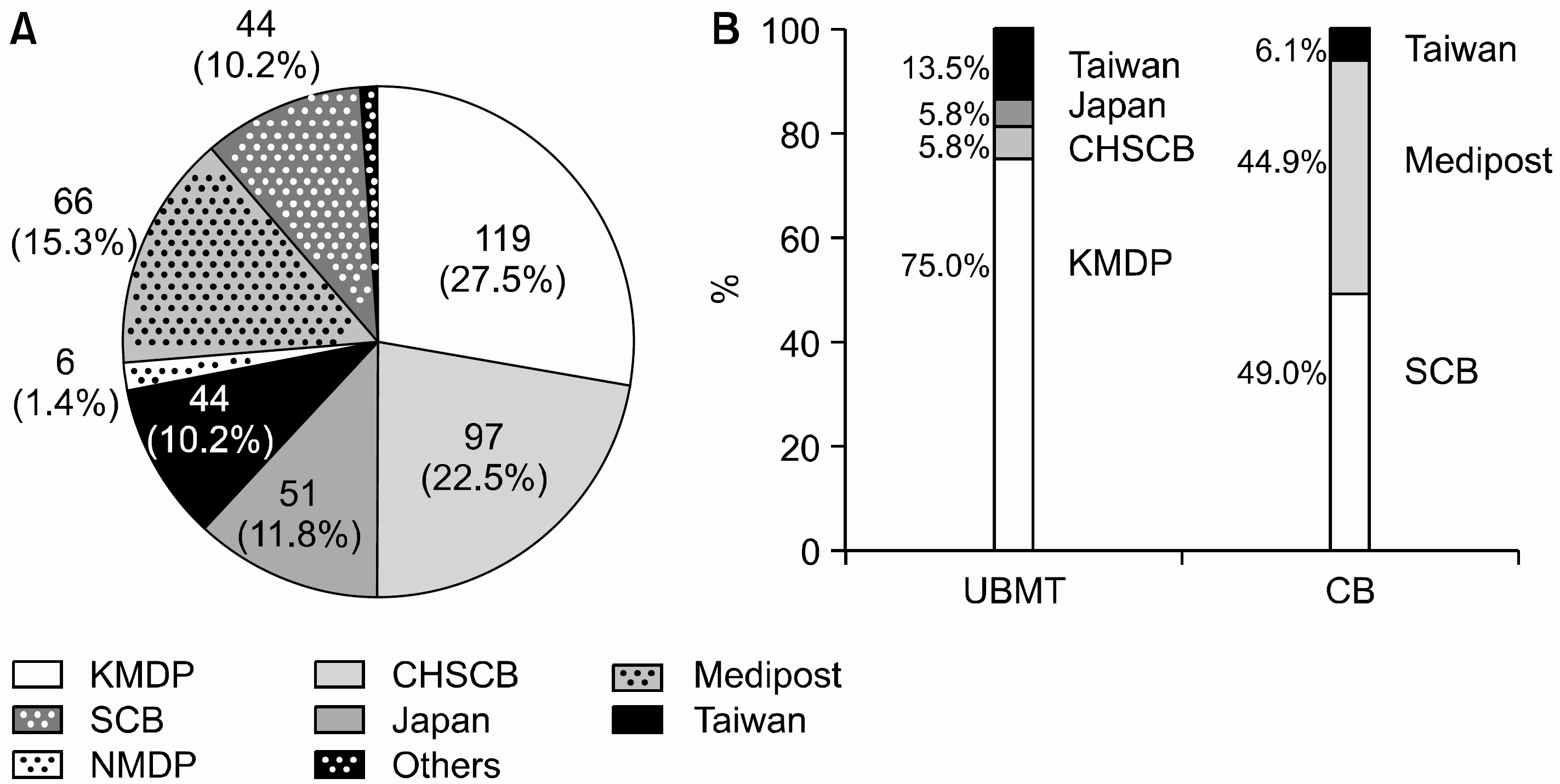
Seven hundred forty eight children (male to female ratio = 1.4:1) were enrolled. The median age was 6.1 years old (8 days~28.8 years old). Malignant diseases consisted of 695 cases (92.9%), and among them almost half were hematologic malignancies. The participating members speculated that HSCTs should be indicated in 285 children (38.1%) which included 209 allogeneic, and 76 autologous transplants. In reality, however, allogeneic HSCTs were performed only in 140 children (67.0%) with the median interval of 5.9 month, and autologous transplants in 44 children (57.9%) with 8.3 month. In autologous setting, all the patients received peripheral blood stem cells (PBSCs), whereas bone marrow (61%), cord blood (34%), and PBSC (5%) were used in allogeneic HSCTs. Donor types were as follows: unrelated donor (37%), cord blood (34%), sibling donor (25%), and family (4%). The reasons for not performing HSCTs were unfavorable disease status or death, no availability of suitable donor, economical situation, and refusal by parental preferences. Under the strict insurance regulations, many transplants were not covered by insurance. More autologous transplants were performed without insurance coverage than allogeneic HSCTs (P=0.013). Those cases were advanced cases and HLA mismatch transplants for allogeneic setting, and relatively rare diseases still awaiting favorable results of transplants for autologous setting.
CONCLUSION
HSCTs are essential part of treatment strategies for children with various diseases. Unfortunately, however, a third of patients who were in need of transplants did not receive HSCTs due to various reasons. It is necessary to expand unrelated donor pool or cord blood banks for the cases lacking HLA-identical sibling donors. Also medical insurances should cover HSCTs for rare diseases as well as for less favorable but novel situations where there are no suitable alternatives.
MeSH Terms
-
Autografts
Bone Marrow
Child*
Disulfiram
Female
Fetal Blood
Hematologic Diseases
Hematologic Neoplasms
Hematopoietic Stem Cell Transplantation*
Hematopoietic Stem Cells*
Humans
Insurance
Insurance Coverage
Parents
Rare Diseases
Siblings
Social Control, Formal
Stem Cells
Tissue Donors
Unrelated Donors
Surveys and Questionnaires
Disulfiram
Figure
Reference
-
1). Bach FH., Albertini RJ., Joo P., Anderson JL., Bortin MM. Bone-marrow transplantation in a patient with Wiskott-Aldrich syndrome. Lancet. 1968. 2:1364–6.2). Santos GW. Bone marrow transplantation in hematologic malignancies: current status. Cancer. 1990. 65:786–91.3). Bortin MM., Horowitz MM., Rimm AA. Increasing utilization of allogeneic bone marrow transplantation: results of the 1988~1990 survey. Ann Intern Med. 1992. 116:505–12.4). Steward CG., Jarisch A. Hematopoietic stem cell transplantation for genetic disorders. Arch Dis Child. 2005. 90:1259–63.5). Petersdorf EW., Malkki M. Human leukocyte antigen matching in unrelated donor hematopoietic cell transplantation. Semin Hematol. 2005. 42:76–84.
Article6). Iwasaki T. Recent advances in the treatment of graft-versus-host disease. Clin Med Res. 2004. 2:243–52.
Article7). Dale DC. Colony-stimulating factors for the management of neutropenia in cancer patients. Drugs. 2002. 62(suppl 1):1–15.
Article8). Lam HH., Althaus BL. Antifungal prophylaxis in bone marrow transplant. Ann Pharmacother. 1995. 29:921–4.
Article9). McFarland W., Granville NB., Dameshek W. Autologous bone marrow infusion as an adjunct in the therapy of malignant disease. Blood. 1959. 14:503–21.10). To LB., Haylock DN., Kimber RJ., Juttner CA. High levels of circulating haematopoietic stem cells in very early remission from acute non-lymphoblastic leukemia and their collection and cryopreservation. Br J Haematol. 1984. 58:399–410.11). Gluckman E., Broxmeyer HA., Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989. 321:1174–8.
Article12). Gluckman E., Rocha V., Chevret S. Results of unrelated umbilical cord blood hematopoietic stem cell transplant. Transfus Clin Biol. 2001. 8:146–54.
Article13). Diez-Campelo M., Perez-Simon JA., Ocio EM, et al. CD34+ cell dose and outcome of patients undergoing reduced-intensity-conditioning allogeneic peripheral blood stem cell transplantation. Leuk Lymphoma. 2005. 46:177–83.14). Mielke S., Solomon SR., Barrett AJ. Selective depletion strategies in allogeneic stem cell transplantation. Cytotherapy. 2005. 7:109–15.
Article15). Horowitz MM., Rowlings PA. An update from the international bone marrow transplant registry and the autologous blood and marrow transplant registry on current activity in hematopoietic stem cell transplantation. Curr Opin Hematol. 1997. 4:395–400.
Article16). Jeong DC., Kang IJ., Koo HH, et al. Epidemiology and clinical outcomes in children with aplastic anemia in Korea: retrospective study. Korean J Pediatr Hematol-Oncol. 2004. 11:137–52.17). Rosti G., Ferrante P., Ledermann J, et al. High-dose chemotherapy for solid tumors: results of the EBMT. Crit Rev Oncol Hematol. 2002. 41:129–40.
Article18). Michel G., Rocha V., Chevret S, et al. Unrelated cord blood transplantation for childhood acute myeloid leukemia: a Eurocord Group analysis. Blood. 2003. 102:4290–7.
Article19). Bradley MB., Cairo MS. Cord blood immunology and stem cell transplantation. Hum Immunol. 2005. 66:431–46.
Article20). Hung GY., Chiou TJ., Chang CY., Teng HW., Chen PM., Hwanga B. Double-unit unrelated cord blood transplantation for chronic myelogenous leukemia. Int J Hematol. 2005. 82:159–61.
Article21). Sugarman J., Kaalund V., Kodish E, et al. Ethical issues in umbilical cord blood banking. Working Group on Ethical Issues in Umbilical Cord Blood Banking. JAMA. 1997. 278:938–43.
Article22). Warwick R., Armitage S. Cord blood banking. Best Pract Res Clin Obstet Gynaecol. 2004. 18:995–1011.
Article23). Meyers PA. High-dose therapy with autologous stem cell rescue for pediatric sarcomas. Curr Opin Oncol. 2004. 16:120–5.
Article24). Campbell AD., Cohn SL., Reynolds M, et al. Treatment of relapsed Wilms' tumor with high-dose therapy and autologous hematopoietic stem-cell rescue: the experience at Children's Memorial Hospital. J Clin Oncol. 2004. 22:2885–90.
Article25). Revision of criteria of insurance coverage for hematopoietic stem cell transplantation. Korean Ministry of Health & Welfare. Notice No.2005–65.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Hematopoietic Stem Cell Transplantation
- Proposal for an Ideal Management System for Hematopoietic Stem Cells in Korea
- Hematopoietic Stem Cell Transplantation in Inborn Error of Metabolism
- Educational Needs of Families of Children Undergoing Hematopoietic Stem Cell Transplantation