Immune Netw.
2016 Jun;16(3):165-175. 10.4110/in.2016.16.3.165.
Immunomodulatory Effects of Ambroxol on Airway Hyperresponsiveness and Inflammation
- Affiliations
-
- 1Division of Cell Biology, Department of Pediatrics, National Jewish Health, Denver, CO 80206, U.S.A. takedak@njhealth.org
- 2Department of Hematology, Oncology, Allergy and Respiratory Medicine, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, Okayama 700-8558, Japan.
- KMID: 2299790
- DOI: http://doi.org/10.4110/in.2016.16.3.165
Abstract
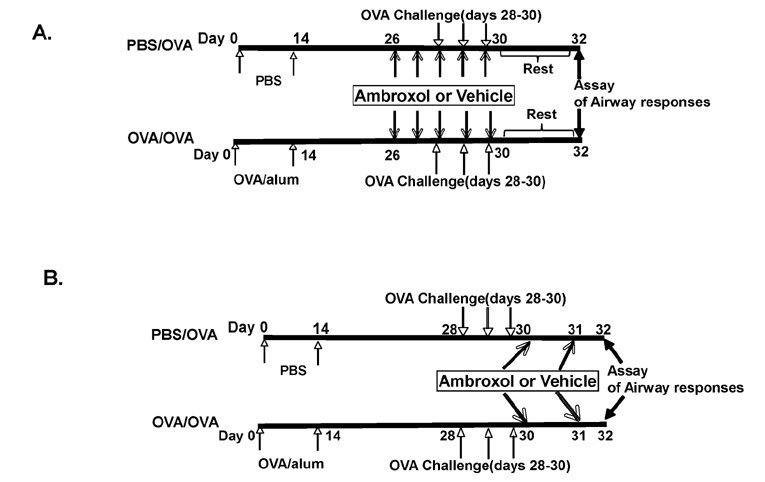
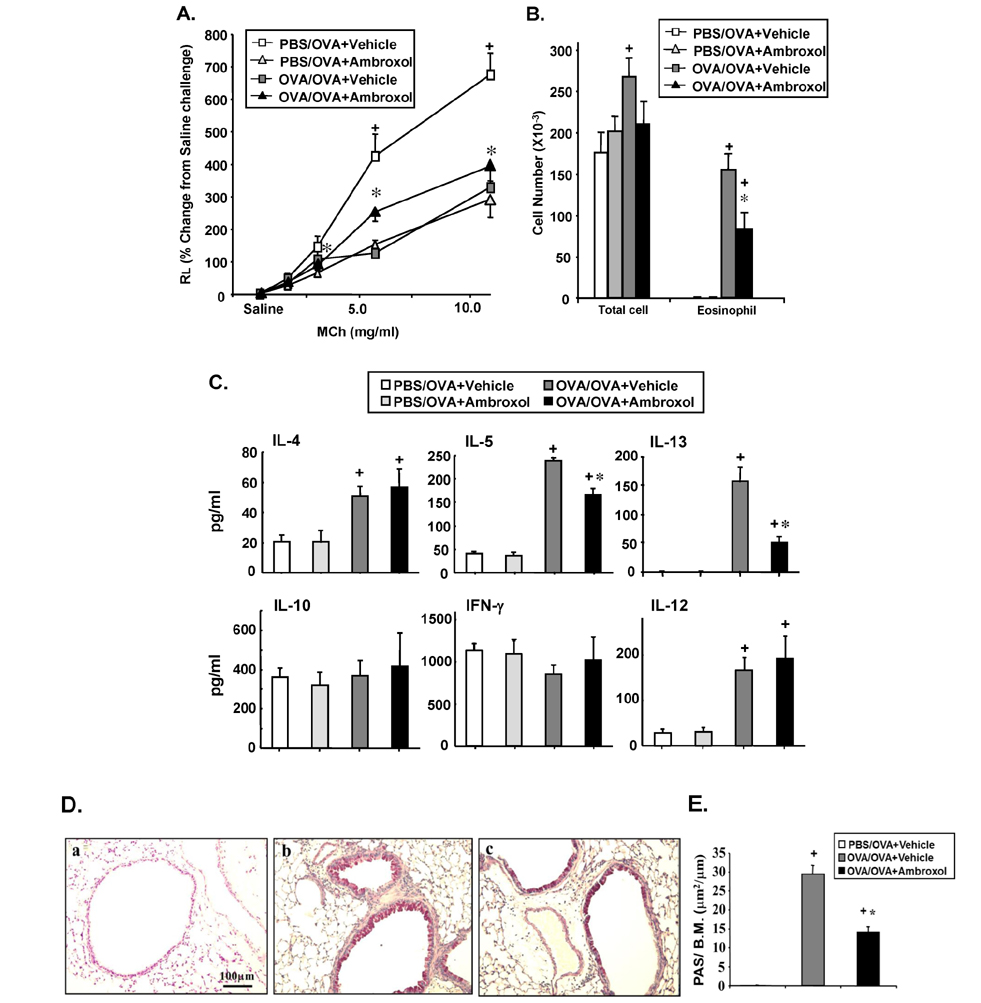
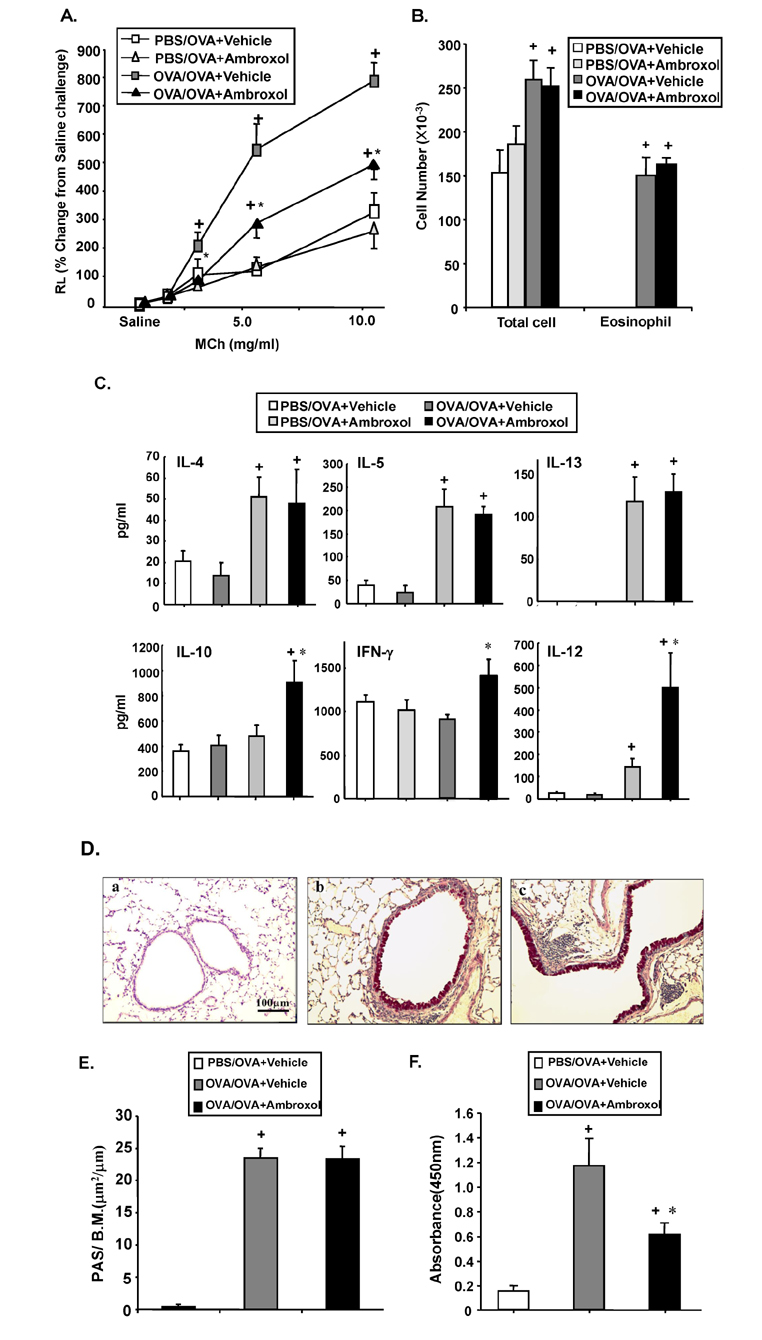
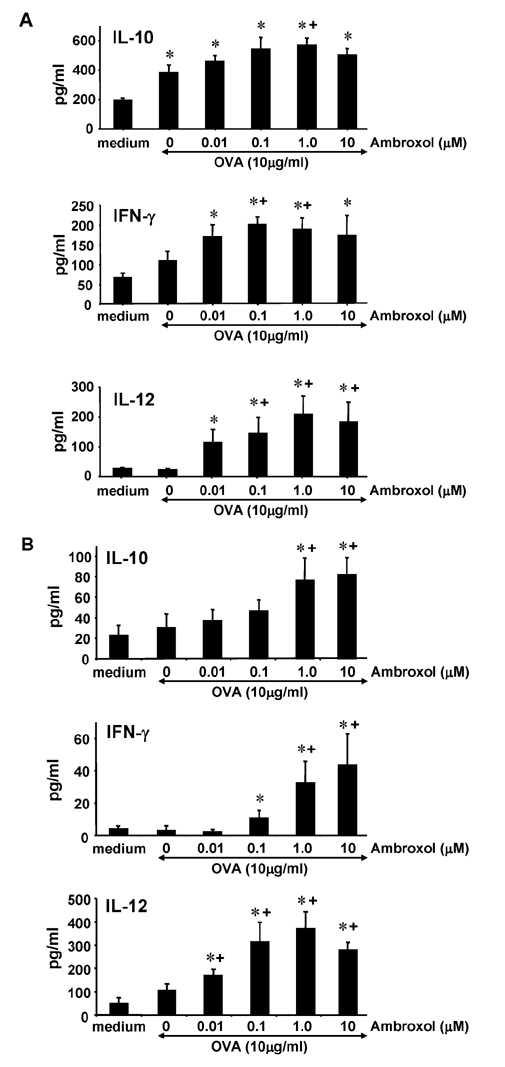
- Ambroxol is used in COPD and asthma to increase mucociliary clearance and regulate surfactant levels, perhaps through anti-oxidant and anti-inflammatory activities. To determine the role and effect of ambroxol in an experimental model of asthma, BALB/c mice were sensitized to ovalbumin (OVA) followed by 3 days of challenge. Airway hyperresponsiveness (AHR), lung cell composition and histology, and cytokine and protein carbonyl levels in bronchoalveolar lavage (BAL) fluid were determined. Ambroxol was administered either before the first OVA challenge or was begun after the last allergen challenge. Cytokine production levels from lung mononuclear cells (Lung MNCs) or alveolar macrophages (AM) were also determined. Administration of ambroxol prior to challenge suppressed AHR, airway eosinophilia, goblet cell metaplasia, and reduced inflammation in subepithelial regions. When given after challenge, AHR was suppressed but without effects on eosinophil numbers. Levels of IL-5 and IL-13 in BAL fluid were decreased when the drug was given prior to challenge; when given after challenge, increased levels of IL-10 and IL-12 were detected. Decreased levels of protein carbonyls were detected in BAL fluid following ambroxol treatment after challenge. In vitro, ambroxol increased levels of IL-10, IFN-γ, and IL-12 from Lung MNCs and AM, whereas IL-4, IL-5, and IL-13 production was not altered. Taken together, ambroxol was effective in preventing AHR and airway inflammation through upregulation of Th1 cytokines and protection from oxidative stress in the airways.
Keyword
MeSH Terms
-
Ambroxol*
Animals
Asthma
Bronchoalveolar Lavage
Cytokines
Eosinophilia
Eosinophils
Goblet Cells
In Vitro Techniques
Inflammation*
Interleukin-10
Interleukin-12
Interleukin-13
Interleukin-4
Interleukin-5
Lung
Macrophages, Alveolar
Metaplasia
Mice
Models, Theoretical
Mucociliary Clearance
Neutrophils
Ovalbumin
Ovum
Oxidative Stress
Pulmonary Disease, Chronic Obstructive
Up-Regulation
Ambroxol
Cytokines
Interleukin-10
Interleukin-12
Interleukin-13
Interleukin-4
Interleukin-5
Ovalbumin
Figure
Reference
-
1. Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2010; 65:152–167.
Article2. Thomas M. Why aren't we doing better in asthma: time for personalised medicine. NPJ Prim Care Respir Med. 2015; 25:15004.
Article3. Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway mucus: From production to secretion. Am J Respir Cell Mol Biol. 2006; 34:527–536.4. Lai HY, Rogers DF. Mucus hypersecretion in asthma: intracellular signaling pathways as targets for pharmacotherapy. Curr Opin Allergy Clin Immunol. 2010; 10:67–76.
Article5. Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001; 108:594–601.
Article6. Morcillo EJ, Cortijo J. Mucus and MUC in asthma. Curr Opin Pulm Med. 2006; 12:1–6.
Article7. Ishibashi Y, Kobayashi F, Idesawa A, Taniguchi A, Matsuzawa S. Effects of carbocisteine on altered activities of glycosidase and glycosyltransferase and expression of Muc5ac in SO2-exposed rats. Eur J Pharmacol. 2004; 487:7–15.
Article8. Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: Central mediator of allergic asthma. Science. 1998; 282:2258–2261.
Article9. Takeyama K, Fahy JV, Nadel JA. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med. 2001; 163:511–516.
Article10. Malerba M, Ponticiello A, Radaeli A, Bensi G, Grassi V. Effect of twelve-months therapy with oral ambroxol in preventing exacerbations in patients with COPD. Double-blind, randomized, multicenter, placebo-controlled study (the AMETHIST Trial). Pulm Pharmacol Ther. 2004; 17:27–34.
Article11. Ratjen F, Wonne R, Posselt HG, Stover B, Hofmann D, Bender SW. A double-blind placebo controlled trial with oral ambroxol and N-acetylcysteine for mucolytic treatment in cystic fibrosis. Eur J Pediatr. 1985; 144:374–378.
Article12. Malerba M, Ragnoli B. Ambroxol in the 21st century: Pharmacological and clinical update. Expert Opin Drug Metab Toxicol. 2008; 4:1119–1129.
Article13. Paleari D, Rossi GA, Nicolini G, Olivieri D. Ambroxol: a multifaceted molecule with additional therapeutic potentials in respiratory disorders of childhood. Expert Opin Drug Discov. 2011; 6:1203–1214.
Article14. Melillo G, Cocco G. Ambroxol decreases bronchial hyperreactivity. Eur J Respir Dis. 1986; 69:316–320.15. Spicak V, Kacirek S, Pohunek P. Treatment of bronchial obstruction in asthmatic children. Bull Eur Physiopathol Respir. 1987; 10:107s–109s.16. Huang J, Xu J, Tian L, Zhong L. A thioredoxin reductase and/or thioredoxin system-based mechanism for antioxidant effects of ambroxol. Biochimie. 2014; 97:92–103.
Article17. Seifart C, Clostermann U, Seifart U, Muller B, Vogelmeier C, von Wichert P, Fehrenbach H. Cellspecific modulation of surfactant proteins by ambroxol treatment. Toxicol Appl Pharmacol. 2005; 203:27–35.
Article18. Seagrave J, Albrecht HH, Hill DB, Rogers DF, Solomon G. Effects of guaifenesin, N-acetylcysteine, and ambroxol on MUC5AC and mucociliary transport in primary differentiated human tracheal-bronchial cells. Respir Res. 2012; 13:98.
Article19. Laoag-Fernandez JB, Fernandez AM, Maruo T. Antenatal use of ambroxol for the prevention of infant respiratory distress syndrome. J Obstet Gynaecol Res. 2000; 26:307–312.
Article20. Gonzalez Garay AG, Reveiz L, Velasco Hidalgo L, Solis Galicia C. Ambroxol for women at risk of preterm birth for preventing neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2014; 10:CD009708.
Article21. Su X, Wang L, Song Y, Bai C. Inhibition of inflammatory responses by ambroxol, a mucolytic agent, in a murine model of acute lung injury induced by lipopolysaccharide. Intensive Care Med. 2004; 30:133–140.
Article22. Gibbs BF. Differential modulation of IgE-dependent activation of human basophils by ambroxol and related secretolytic analogues. Int J Immunopathol Pharmacol. 2009; 22:919–927.
Article23. Dong C, Wang G, Li B, Xiao K, Ma Z, Huang H, Wang X, Bai C. Anti-asthmatic agents alleviate pulmonary edema by upregulating AQP1 and AQP5 expression in the lungs of mice with OVA-induced asthma. Respir Physiol Neurobiol. 2012; 181:21–28.
Article24. Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med. 1997; 186:449–454.
Article25. Takeda K, Miyahara N, Kodama T, Taube C, Balhorn A, Dakhama A, Kitamura K, Hirano A, Tanimoto M, Gelfand EW. S-carboxymethylcysteine normalises airway responsiveness in sensitised and challenged mice. Eur Respir J. 2005; 26:577–585.
Article26. Ritter C, da Cunha AA, Echer IC, Andrades M, Reinke A, Lucchiari N, Rocha J, Streck EL, Menna-Barreto S, Moreira JC, Dal-Pizzol F. Effects of N-acetylcysteine plus deferoxamine in lipopolysaccharide-induced acute lung injury in the rat. Crit Care Med. 2006; 34:471–477.
Article27. Houtmeyers E, Gosselink R, Gayan-Ramirez G, Decramer M. Effects of drugs on mucus clearance. Eur Respir J. 1999; 14:452–467.
Article28. Himmel W, Hummers-Pradier E, Schumann H, Kochen MM. The predictive value of asthma medications to identify individuals with asthma--a study in German general practices. Br J Gen Pract. 2001; 472:879–883.29. Lemanske RF Jr, Busse WW. Asthma: Factors underlying inception, exacerbation, and disease progression. J Allergy Clin Immunol. 2006; 117:S456–S461.30. O'Byrne PM, Inman MD, Adelroth E. Reassessing the Th2 cytokine basis of asthma. Trends Pharmacol Sci. 2004; 25:244–248.31. Schwarze J, Hamelmann E, Cieslewicz G, Tomkinson A, Joetham A, Bradley K, Gelfand EW. Local treatment with IL-12 is an effective inhibitor of airway hyperresponsiveness and lung eosinophilia after airway challenge in sensitized mice. J Allergy Clin Immunol. 1998; 102:86–93.
Article32. Matsumoto K, Inoue H, Tsuda M, Honda Y, Kibe A, Machida K, Yoshiura Y, Nakanishi Y. Different roles of interleukin-10 in onset and resolution of asthmatic responses in allergen-challenged mice. Respirology. 2005; 10:18–26.
Article33. Ma X, Yan W, Zheng H, Du Q, Zhang L, Ban Y, Li N, Wei F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Res. 2015; 4:pii: F1000 Faculty Rev-1465.
Article34. Bowler RP, Crapo JD. Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol. 2002; 110:349–356.
Article35. Park JW, Taube C, Swasey C, Kodama T, Joetham A, Balhorn A, Takeda K, Miyahara N, Allen CB, Dakhama A, Kim SH, Dinarello CA, Gelfand EW. Complement activation is critical to airway hyperresponsiveness after acute ozone exposure. Am J Respir Crit Care Med. 2004; 169:726–732.
Article36. Comhair SA, Ricci KS, Arroliga M, Lara AR, Dweik RA, Song W, Hazen SL, Bleecker ER, Busse WW, Chung KF, Gaston B, Hastie A, Hew M, Jarjour N, Moore W, Peters S, Teague WG, Wenzel SE, Erzurum SC. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am J Respir Crit Care Med. 2005; 172:306–313.
Article37. Blesa S, Cortijo J, Mata M, Serrano A, Closa D, Santangelo F, Estrela JM, Suchankova J, Morcillo EJ. Oral N-acetylcysteine attenuates the rat pulmonary inflammatory response to antigen. Eur Respir J. 2003; 21:394–400.
Article38. Larsen GL, White CW, Takeda K, Loader JE, Nguyen DD, Joetham A, Groner Y, Gelfand EW. Mice that overexpress Cu/Zn superoxide dismutase are resistant to allergen-induced changes in airway control. Am J Physiol Lung Cell Mol Physiol. 2000; 279:L350–L359.
Article39. Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005; 202:47–59.
Article40. Teramoto S, Suzuki M, Ohga E, Ishii T, Matsui H, Matsuse T, Ouchi Y. Effects of ambroxol on spontaneous or stimulated generation of reactive oxygen species by bronchoalveolar lavage cells harvested from patients with or without chronic obstructive pulmonary diseases. Pharmacology. 1999; 3:135–141.
Article41. Gillissen A, Bartling A, Schoen S, Schultze-Werninghaus G. Antioxidant function of ambroxol in mononuclear and polymorphonuclear cells In vitro. Lung. 1997; 175:235–242.
Article42. Peroni DG, Moser S, Gallo G, Pigozzi R, Tenero L, Zanoni L, Boner AL, Piacentini GL. Ambroxol inhibits neutrophil respiratory burst activated by alpha chain integrin adhesion. Int J Immunopathol Pharmacol. 2013; 26:883–887.
Article43. Chitano P, Di Stefano A, Finotto S, Zavattini G, Maestrelli P, Mapp C, Fabbri LM, Allegra L. Ambroxol inhibits airway hyperresponsiveness induced by ozone in dogs. Respiration. 1989; 55:Suppl 1. 74–78.
Article44. Buss IH, Darlow BA, Winterbourn CC. Elevated protein carbonyls and lipid peroxidation products correlating with myeloperoxidase in tracheal aspirates from premature infants. Pediatr Res. 2000; 47:640–645.
Article45. Frossi B, De Carli M, Piemonte M, Pucillo C. Oxidative microenvironment exerts an opposite regulatory effect on cytokine production by Th1 and Th2 cells. Mol Immunol. 2008; 45:58–64.
Article46. Obata F, Hoshino A, Toyama A. Hydrogen peroxide increases interleukin-12 p40/p70 molecular ratio and induces Th2-predominant responses in mice. Scand J Immunol. 2006; 63:125–130.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem cell therapy in animal models of allergic airway diseases
- Effects of Ambroxol, S-carboxymethylcysteine, Dextromethorphan and Noscapine on Mucin Release from Airway Goblet Cells
- Effects of CpG-oligodeoxynucleotides in Chronic Inflammation and Remodeling of Airway in a Murine Model of Bronchial Asthma
- The Preventive Effect of Antenatal Administration of Ambroxol on the Neonatal Respiratory Distress Syndrome
- The Comparison Study of teh Effect of Ambroxol on Prevention of Infantile Respiratory Distress Syndrome in Preterm Delivery