Korean Circ J.
2011 Oct;41(10):603-611. 10.4070/kcj.2011.41.10.603.
Survival, Exercise Capacity, and Left Ventricular Remodeling in a Rat Model of Chronic Mitral Regurgitation: Serial Echocardiography and Pressure-Volume Analysis
- Affiliations
-
- 1Department of Internal Medicine, Cardiovascular Center, Seoul National University College of Medicine, Seoul, Korea. kimdamas@snu.ac.kr
- KMID: 2297920
- DOI: http://doi.org/10.4070/kcj.2011.41.10.603
Abstract
- BACKGROUND AND OBJECTIVES
The aims of this study were to establish a reliable model of chronic mitral regurgitation (MR) in rats and verify the pathophysiological features of this model by evaluating cardiac function using serial echocardiography and a pressure-volume analysis.
MATERIALS AND METHODS
MR was created in 37 Sprague-Dawley rats by making a hole with a 23 gauge needle on the mitral leaflet through the left ventricular (LV) apex under the guidance of transesophageal echocardiography.
RESULTS
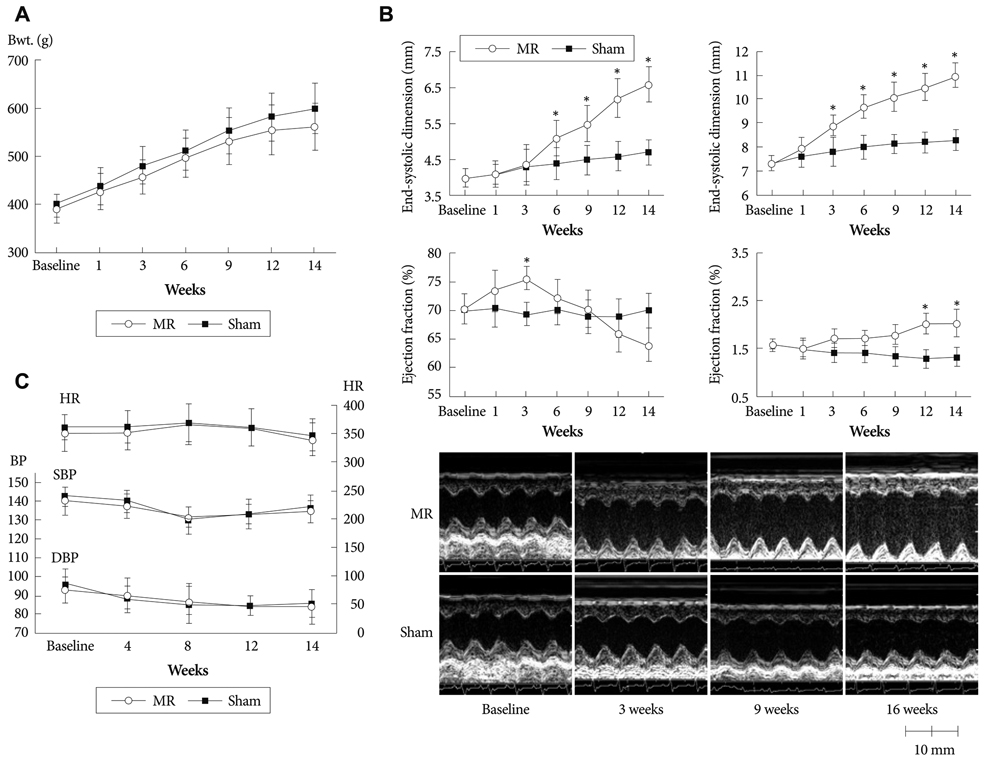
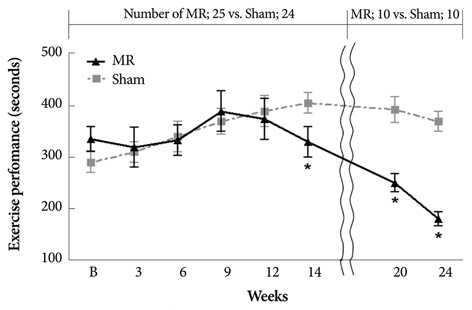
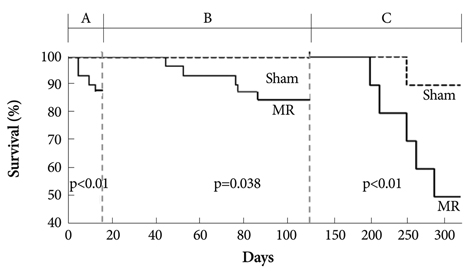
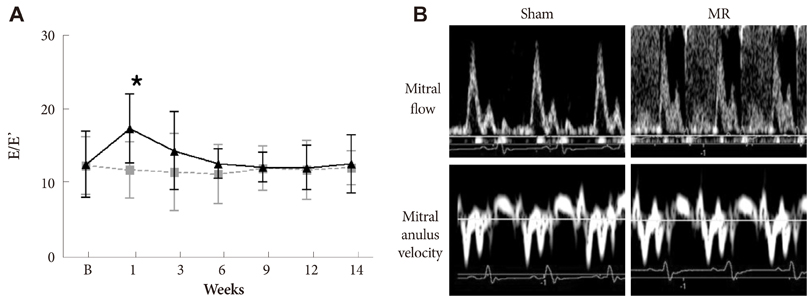
Serial echocardiograms revealed that the LV began to dilate immediately after the MR operation and showed progressive dilation until the 14th week (LV end-systolic dimension at 14 weeks, 4.71+/-0.25 mm vs. 6.81+/-0.50 mm for sham vs. MR, p<0.01; LV end-diastolic dimension, 8.32+/-0.42 mm vs. 11.01+/-0.47 mm, p<0.01). The LV ejection fraction tended to increase immediately after the MR operation but started to decrease thereafter and showed a significant difference with the sham group from the 14th week (70.0+/-2.2% vs. 62.1+/-3.1% for sham vs. MR). In a pressure-volume analysis performed at the 14th week, the LV end-systolic pressure-volume relationship and +dp/dt decreased significantly in the MR group. A serial treadmill test revealed that exercise capacity remained in the normal range until the 14th week when it began to decrease (exercise duration, 406+/-45 seconds vs. 330+/-27 seconds, p<0.01). A pathological analysis showed no significance difference in interstitial fibrosis between the two groups.
CONCLUSION
We established a small animal model of chronic MR and verified its pathophysiological features. This model may provide a useful tool for future research on MR and volume overload heart failure.
MeSH Terms
Figure
Cited by 2 articles
-
Hemodynamic and Histopathologic Benefits of Early Treatment with Macitentan in a Rat Model of Pulmonary Arterial Hypertension
Kyung-Hee Kim, Hyung-Kwan Kim, Stephen Y. Chan, Yong-Jin Kim, Dae-Won Sohn
Korean Circ J. 2018;48(9):839-853. doi: 10.4070/kcj.2017.0394.Differential Transcriptome Profile and Exercise Capacity in Cardiac Remodeling by Pressure Overload versus Volume Overload
Kyung-Hee Kim, Hyue-Mee Kim, Jin-Sik Park, Yong-Jin Kim
J Cardiovasc Imaging. 2019;27(1):50-63. doi: 10.4250/jcvi.2019.27.e4.
Reference
-
1. Eriksson H. Heart failure: a growing public health problem. J Intern Med. 1995. 237:135–141.2. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007. 93:1137–1146.3. Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail. 2009. 2:138–144.4. Monnet E, Chachques JC. Animal models of heart failure: what is new? Ann Thorac Surg. 2005. 79:1445–1453.5. Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008. 3:1422–1434.6. Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999. 83:897–902. Erratum in: Am J Cardiol 1999;84:1143.7. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JJ, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006. 368:1005–1011.8. Zheng J, Chen Y, Pat B, et al. Microarray identifies extensive downregulation of noncollagen extracellular matrix and profibrotic growth factor genes in chronic isolated mitral regurgitation in the dog. Circulation. 2009. 119:2086–2095.9. Mishra YK, Mittal S, Jaguri P, Trehan N. Coapsys mitral annuloplasty for chronic functional ischemic mitral regurgitation: 1-year results. Ann Thorac Surg. 2006. 81:42–46.10. Pu M, Gao Z, Li J, Sinoway L, Davidson WR Jr. Development of a new animal model of chronic mitral regurgitation in rats under transesophageal echocardiographic guidance. J Am Soc Echocardiogr. 2005. 18:468–474.11. Reffelmann T, Kloner RA. Transthoracic echocardiography in rats: evalution of commonly used indices of left ventricular dimensions, contractile performance, and hypertrophy in a genetic model of hypertrophic heart failure (SHHF-Mcc-facp-Rats) in comparison with Wistar rats during aging. Basic Res Cardiol. 2003. 98:275–284.12. Litwin SE, Katz SE, Weinberg EO, Lorell BH, Aurigemma GP, Douglas PS. Serial echocardiographic-Doppler assessment of left ventricular geometry and function in rats with pressure-overload hypertrophy: chronic angiotensin-converting enzyme inhibition attenuates the transition to heart failure. Circulation. 1995. 91:2642–2654.13. Helmcke F, Nanda NC, Hsiung MC, et al. Color Doppler assessment of mitral regurgitation with orthogonal planes. Circulation. 1987. 75:175–183.14. Chang SA, Kim YJ, Lee HW, et al. Effect of rosuvastatin on cardiac remodeling, function, and progression to heart failure in hypertensive heart with established left ventricular hypertrophy. Hypertension. 2009. 54:591–597.15. Lips DJ, van der Nagel T, Steendijk P, et al. Left ventricular pressure-volume measurements in mice: comparison of closed-chest versus open-chest approach. Basic Res Cardiol. 2004. 99:351–359.16. Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005. 289:H501–H512.17. Ichihara S, Senbonmatsu T, Price E Jr, Ichiki T, Gaffney FA, Inagami T. Angiotensin II type 2 receptor is essential for left ventricular hypertrophy and cardiac fibrosis in chronic angiotensin II-induced hypertension. Circulation. 2001. 104:346–351.18. Monnet E, Chachques JC. Animal models of heart failure: What is new? Ann Thorac Surg. 2005. 79:1445–1453.19. Daimon M, Shiota T, Gillinov AM, et al. Percutaneous mitral valve repair for chronic ischemic mitral regurgitation: a real-time three-dimensional echocardiographic study in an ovine model. Circulation. 2005. 111:2183–2189.20. Chaput M, Handschumacher MD, Guerrero JL, et al. Mitral leaflet adaptation to ventricular remodeling: prospective changes in a model of ischemic mitral regurgitation. Circulation. 2009. 120:11 Suppl. S99–S103.21. Pu M, Gao Z, Zhang X, et al. Impact of mitral regurgitation on left ventricular anatomic and molecular remodeling and systolic function: implication for outcome. Am J Physiol Heart Circ Physiol. 2009. 296:H1727–H1737.22. Zile MR, Tomita M, Nakano K, et al. Effects of left ventricular volume overload produced by mitral regurgitation on diastolic function. Am J Physiol. 1991. 261:H1471–H1480.23. Corin WJ, Murakami T, Monrad ES, Hess OM, Krayenbuehl HP. Left ventricular passive diastolic properties in chronic mitral regurgitation. Circulation. 1991. 83:797–807.24. Ryan TD, Rothstein EC, Aban I, et al. Left ventricular eccentric remodeling and matrix loss are mediated by bradykinin and precede cardiomyocyte elongation in rats with volume overload. J Am Coll Cardiol. 2007. 49:811–821.25. Weber KT, Pick R, Silver MA, et al. Fibrillar collagen and remodeling of dilated canine left ventricle. Circulation. 1990. 82:1387–1401.26. Michel JB, Salzmann JL, Ossondo Nlom M, Bruneval P, Barres D, Camilleri JP. Morphometric analysis of collagen network and plasma perfused capillary bed in the myocardium of rats during evolution of cardiac hypertrophy. Basic Res Cardiol. 1986. 81:142–154.27. Pacher P, Mabley JG, Liaudet L, et al. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol. 2004. 287:H2132–H2137.28. Bátkai S, Pacher P, Osei-Hyiaman D, et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004. 110:1996–2002.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of Mitral Valve Complex by Three-Dimensional Echocardiography: Therapeutic Strategy for Functional Mitral Regurgitation
- Comparison of Postoperative LV Function after Mitral Valve Replacement and Predictor of Postoperative LV Function in Chronic Mitral Regurgitation
- The Influence of the Left Ventricular Geometry on the Left Atrial Size and Left Ventricular Filling Pressure in Hypertensive Patients, as Assessed by Echocardiography
- Preoperative Factors Affecting the Outcome of Mitral Valve Replacement in Patients with Chronic Mitral Regurgitation
- Echocardiographic Assessment of Mitral Valve Regurgitation