Korean J Urol.
2006 Nov;47(11):1197-1203. 10.4111/kju.2006.47.11.1197.
The Effect of Long-term Immobilization Stress on Spermatogenesis and Testosterone Production
- Affiliations
-
- 1Departments of Urology and Anatomy, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2294279
- DOI: http://doi.org/10.4111/kju.2006.47.11.1197
Abstract
- Purpose
The aim of this study was to investigate the effects of long-term immobilization stress on spermatogenesis and testosterone production in a rat model.
Materials and Methods
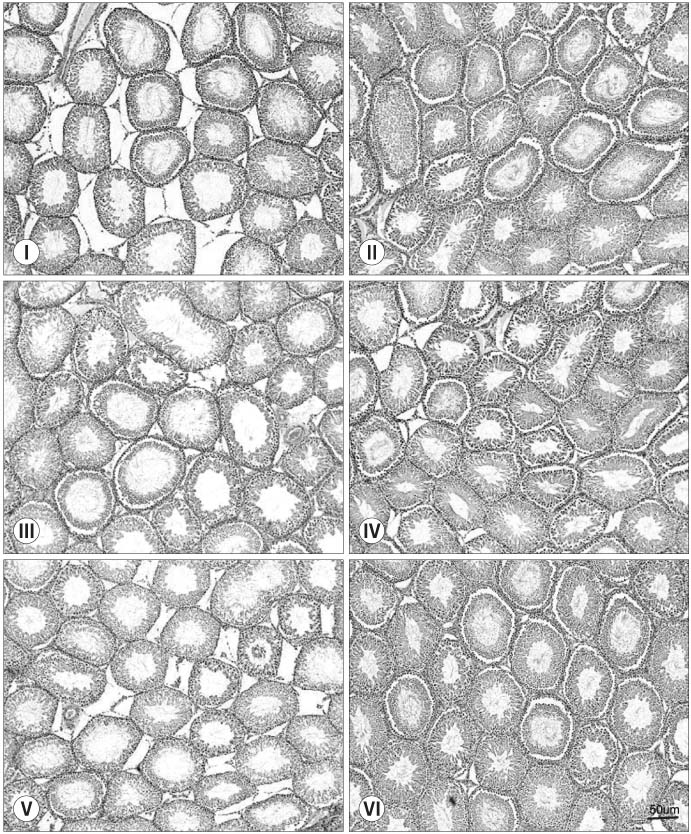
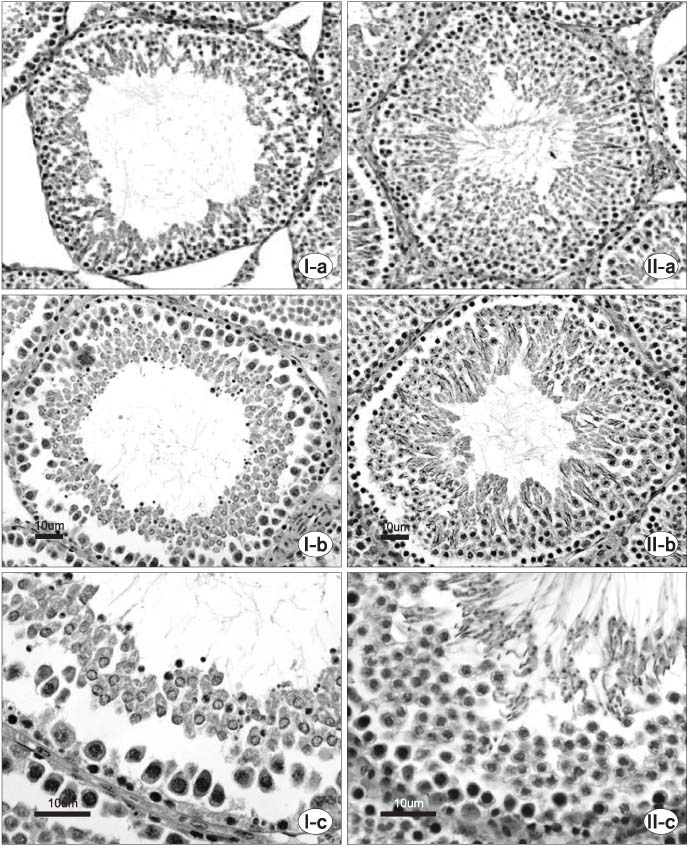
Thirty randomly selected adult male rats were divided into 6 groups: immobilization stress groups (I, III, V) and control groups (II, IV, VI). In the immobilization stress groups, 15 rats were immobilized in a steel cage for 6 hours per day for 14 days. Groups I and II were sacrificed just after finishing the immobilization session. Groups III and IV were sacrificed after a 1 week resting period. Groups V and VI were sacrificed after a 2 week resting period. The serum concentrations of corticosterone, luteinising hormone (LH), follicle stimulating hormone (FSH) and testosterone were measured. Specimens of the testis were stained with hematoxylin-eosin and Masson's trichrome.
Results
Following 2 weeks of immobilization, the serum concentration of corticosterone was significantly increased; whereas, the serum concentrations of LH and testosterone were decreased. However, the serum FSH concentration remained unchanged. After the 1 week resting period, there were significant recoveries in the serum concentrations of corticosterone, LH and testosterone. From the histology of the immobilization group, the mean testicular biopsy score (Johnsen score) was significantly decreased, but the mean value of the seminiferous tubule luminal diameter was significantly increased; whereas, that of the seminiferous tubule diameter remained unchanged. These changes slowly recovered after the resting period.
Conclusions
These results suggest that the exposure to long-term immobilization impairs spermatogenesis and androgenic testicular functions in rats.
Keyword
MeSH Terms
Figure
Reference
-
1. Park NC, Park YS, Hwang KH, Chung MK, Yoon JB. Male infertility: the clinicostatistical analysis of recent 10 years cumulative data. Korean J Urol. 1996. 37:939–946.2. Hassan MA, Killick SR. Negative life style is associated with a significant reduction in fecundity. Fertil Steril. 2004. 81:384–392.3. Magnusdottir EV, Thorsteinsson T, Thorsteinsdottir S, Heimisdottir M, Olafsdottir K. Persistent organochlorines, sedentary occupation, obesity and human male subfertility. Hum Reprod. 2005. 20:208–215.4. Thonneau P, Ducot B, Bujan L, Mieusset R, Spira A. Effect of male occupational heat exposure on time to pregnancy. Int J Androl. 1997. 20:274–278.5. Sheynkin Y, Jung M, Yoo P, Schulsinger D, Komaroff E. Increase in scrotal temperature in laptop computer users. Hum Reprod. 2005. 20:452–455.6. Hafez B, Hafez ES. Stress/aging: endocrine profiles/reproductive dysfunction in men. Arch Androl. 2004. 50:207–238.7. Orr TE, Mann DR. Role of glucocorticoids in the stress-induced suppression of testicular steroidogenesis in adult male rats. Horm Behav. 1992. 26:350–363.8. Fenske M. Role of cortisol in the ACTH-induced suppression of testicular steroidogenesis in guinea pigs. J Endocrinol. 1997. 154:407–414.9. Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003. 463:235–272.10. Suter DE, Schwartz NB, Ringstrom SJ. Dual role of glucocorticoids in regulation of pituitary content and secretion of gonadotropin. Am J Physiol. 1998. 254:595–600.11. Charpenet G, Tache Y, Bernier M, Ducharme JR, Collu R. Stress-induced testicular hyposensitivity to gonadotropin in rats. Role of the pituitary gland. Biol Reprod. 1982. 27:616–623.12. Orr TE, Taylor MF, Bhattacharyya AK, Collins DC, Mann DR. Acute immobilization stress disrupts testicular steroidogenesis in adult male rats by inhibiting the activities of 17a-hydroxylase and 17,20-lyase without affecting the binding of LH/hCG receptors. J Androl. 1994. 15:302–308.13. Gao HB, Tong MH, Hu YQ, Guo QS, Ge R, Hardy MP. Glucocorticoid induces apoptosis in rat leydig cells. Endocrinology. 2002. 143:130–138.14. Cicero TJ, Schainker BA, Meyer ER. Endogenous opioids participate in the regulation of the hypothalamic-pituitary-luteinizing hormone axis and testosterone's negative feedback control of luteinizing hormone. Endocrinology. 1979. 104:1286–1291.15. Anderson ML, Bignotto M, Machado RB, Tufik S. Different stress modalities result in distinct steroid hormone responses by male rats. Braz J Med Biol Res. 2004. 37:791–797.16. Kawazu S, Kishi H, Saita E, Jin W, Suzuki AK, Watanabe G, et al. Inhibin secretion in the golden hamster (Mesocricetus auratus) testis during active and inactive states of spermatogenesis induced by the restriction of photoperiod. J Reprod Dev. 2003. 49:87–97.17. Johnsen SG. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results of 335 hypogonadal males. Hormones. 1970. 1:2–25.18. Suarez M, Fiol de Cuneo M, Vincenti L, Ruiz RD. Changes in corticosterone levels and sperm functional activity by chronic stress in rats. Arch Physiol Biochem. 1996. 104:351–356.19. Tash JS, Johnson DC, Enders GC. Long-term (6-wk) hindlimb suspension inhibits spermatogenesis in adult male rats. J Appl Physiol. 2002. 92:1191–1198.20. Tsuchiya T, Horii I. Different effects of acute and chronic immobilization stress on plasma testosterone levels in male Syrian hamsters. Psychoneuroendocrinology. 1995. 20:95–102.21. Yazawa H, Sasagawa I, Ishigooka M, Nakada T. Effect of immobilization stress on testicular germ cell apoptosis in rats. Hum Reprod. 1999. 14:1806–1810.22. Almeida SA, Petenusci SO, Franci JA, Rosa-e-Silva AA, Carvalho TL. Chronic immobilization-induced stress increases plasma testosterone and delays testicular maturation in pubertal rats. Andrologia. 2000. 32:7–11.23. Fukuda T, Kikuchi M, Kurotaki T, Oyamada T, Yoshikawa H, Yoshikawa T. Age-related changes in the testes of horses. Equine Vet J. 2001. 33:20–25.24. Montella A, Prino A. Morphological findings in cryptorchidism in the adult male. Boll Soc Ital Biol Sper. 1990. 66:215–222.25. Tapanainen JS, Tilly JL, Vihko KK, Hsuch AJ. Hormonal control of apoptotic cell death in the testis: gonadotropins and androgens as testicular cell survival factors. Mol Endocrinol. 1993. 7:643–650.26. Park SH, Park KS, Park YI. Effects of temperature change on heat shock protein 70 expression in rat testes. Korean J Urol. 2003. 44:186–191.27. Sasagawa I, Yazawa H, Suzuki Y, Nakada T. Stress and testicular germ cell apoptosis. Arch Androl. 2001. 47:211–216.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- SPERMATOGENESIS
- Effects of Large Dose Testosterone and Testosterone Combined with HCG on Histological Structure of Mice Testes
- The Spermatogenic Effect of Yacon Extract and Its Constituents and Their Inhibition Effect of Testosterone Metabolism
- The Effect of Spinal Cord Injury on Pituitary-Testicular Hormone Axis in Rats
- Effect of lithium on the Polyamine Response in Brain after Stress