J Gynecol Oncol.
2013 Jul;24(3):265-272. 10.3802/jgo.2013.24.3.265.
Thrombin promotes epithelial ovarian cancer cell invasion by inducing epithelial-mesenchymal transition
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China. weiping_li61@yahoo.cn
- 2Department of Reproductive Medicine, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
- 3Shanghai Key Laboratory of Gynecologic Oncology, Shanghai, China.
- KMID: 2288534
- DOI: http://doi.org/10.3802/jgo.2013.24.3.265
Abstract
OBJECTIVE
Over-expression of thrombin in ovarian cancer cells is associated with poor prognosis. In this study, we investigated the role of thrombin in inducing epithelial-mesenchymal transition (EMT) in SKOV3 epithelial ovarian cancer cells.
METHODS
After thrombin treatment SKOV3 cells were subjected to western blots, reverse-transcription PCR, and enzyme-linked immunosorbent assay to quantify EMT-related proteins, mRNA expression of SMAD2, DKK1, and sFRP1, and the secretion of matrix metalloproteinases (MMPs) and cytokines. Meanwhile, invasion ability was evaluated using transwell assays.
RESULTS
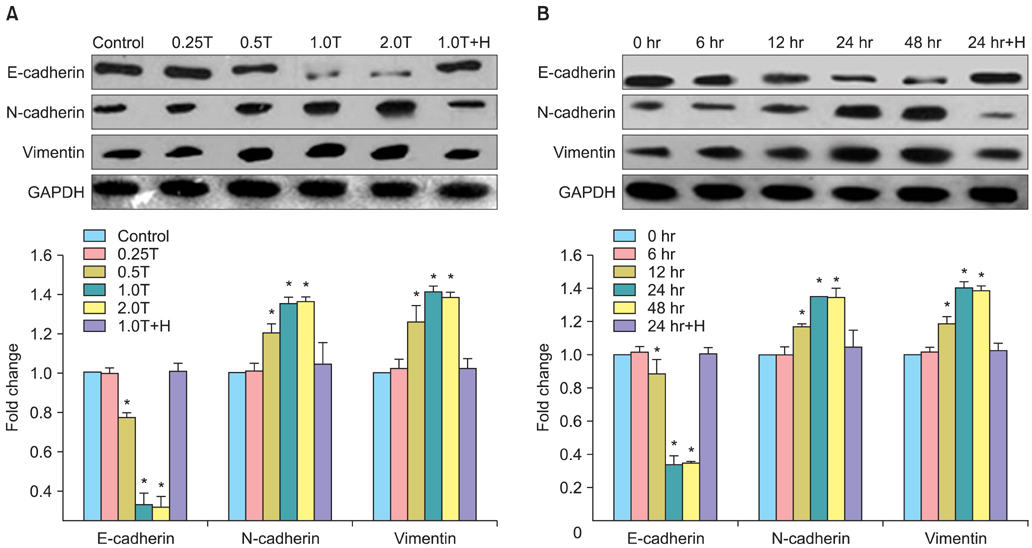
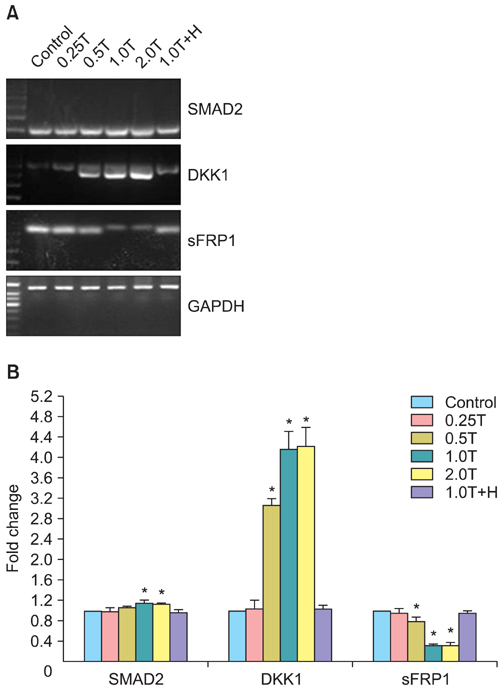
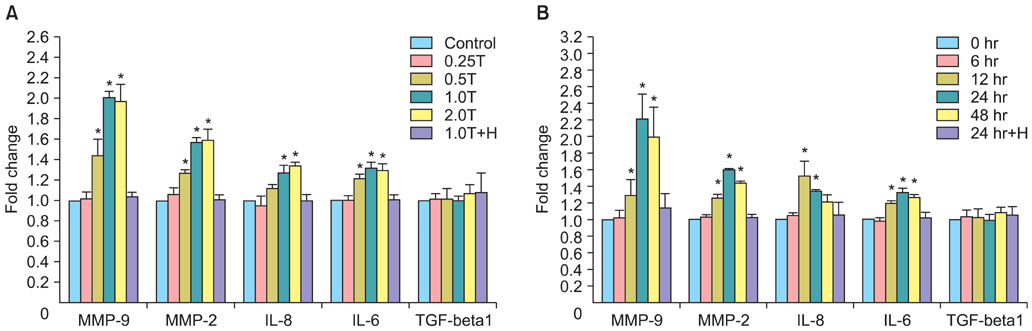
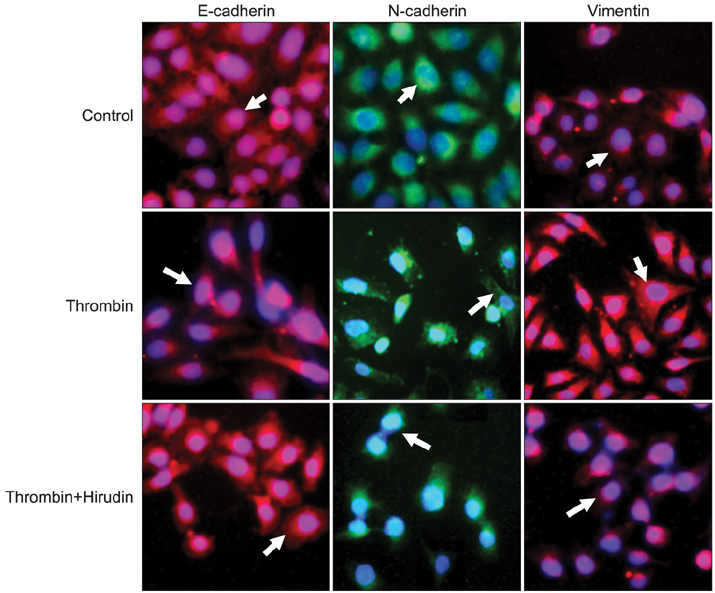
The results indicated a dose- and time-dependent down-regulation of E-cadherin and upregulation of N-cadherin and vimentin in thrombin-treated SKOV3 cells, compared with the thrombin-free control group (p<0.05). There was a dose- and time-dependent increase in the levels of SMAD2 and DKK1 mRNAs and a decrease in the levels of sFRP1 mRNA in thrombin-treated SKOV3 cells compared to control cells (p<0.05). Thrombin-treated SKOV3 cells exhibited increased secretion of MMP-9, MMP-2, interleukin (IL)-8, and IL-6 and increased invasion compared to untreated cells (p<0.05). Thrombin altered the morphology of SKOV3 cells to a spindle-like phenotype. Addition of hirudin to thrombin-treated cells reversed the effects of thrombin.
CONCLUSION
Thrombin induced EMT and promoted the invasion of SKOV3 cells, possibly via distinct signaling pathways. Hirudin inhibited the effects of thrombin, suggesting that anticoagulant therapy could be a novel therapeutic strategy for ovarian carcinoma.
MeSH Terms
-
Blotting, Western
Cadherins
Cytokines
Down-Regulation
Enzyme-Linked Immunosorbent Assay
Epithelial-Mesenchymal Transition
Hirudins
Interleukin-6
Interleukins
Matrix Metalloproteinases
Neoplasms, Glandular and Epithelial
Ovarian Neoplasms
Phenotype
Polymerase Chain Reaction
Prognosis
Proteins
RNA, Messenger
Thrombin
Up-Regulation
Vimentin
Cadherins
Cytokines
Hirudins
Interleukin-6
Interleukins
Matrix Metalloproteinases
Neoplasms, Glandular and Epithelial
Ovarian Neoplasms
Proteins
RNA, Messenger
Thrombin
Vimentin
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71–96.2. Sarojini S, Tamir A, Lim H, Li S, Zhang S, Goy A, et al. Early detection biomarkers for ovarian cancer. J Oncol. 2012; 2012:709049.3. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009; 139:871–890.4. Collino F, Revelli A, Massobrio M, Katsaros D, Schmitt-Ney M, Camussi G, et al. Epithelial-mesenchymal transition of ovarian tumor cells induces an angiogenic monocyte cell population. Exp Cell Res. 2009; 315:2982–2994.5. Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008; 133:704–715.6. Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007; 31:277–283.7. Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009; 27:2059–2068.8. Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007; 39:305–318.9. Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009; 119:1417–1419.10. Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005; 17:548–558.11. Cho EY, Choi Y, Chae SW, Sohn JH, Ahn GH. Immunohistochemical study of the expression of adhesion molecules in ovarian serous neoplasms. Pathol Int. 2006; 56:62–70.12. Franchini M, Montagnana M, Targher G, Manzato F, Lippi G. Pathogenesis, clinical and laboratory aspects of thrombosis in cancer. J Thromb Thrombolysis. 2007; 24:29–38.13. Wang X, Wang E, Kavanagh JJ, Freedman RS. Ovarian cancer, the coagulation pathway, and inflammation. J Transl Med. 2005; 3:25.14. Yousef GM, Polymeris ME, Yacoub GM, Scorilas A, Soosaipillai A, Popalis C, et al. Parallel overexpression of seven kallikrein genes in ovarian cancer. Cancer Res. 2003; 63:2223–2227.15. Egan K, Crowley D, Smyth P, O'Toole S, Spillane C, Martin C, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS One. 2011; 6:e26125.16. Heller RS, Klein T, Ling Z, Heimberg H, Katoh M, Madsen OD, et al. Expression of Wnt, Frizzled, sFRP, and DKK genes in adult human pancreas. Gene Expr. 2003; 11:141–147.17. Zhang G, Kernan KA, Collins SJ, Cai X, Lopez-Guisa JM, Degen JL, et al. Plasmin(ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: role of plasmin-activated signals. J Am Soc Nephrol. 2007; 18:846–859.18. Ando S, Otani H, Yagi Y, Kawai K, Araki H, Fukuhara S, et al. Proteinase-activated receptor 4 stimulation-induced epithelial-mesenchymal transition in alveolar epithelial cells. Respir Res. 2007; 8:31.19. Zain J, Huang YQ, Feng X, Nierodzik ML, Li JJ, Karpatkin S. Concentration-dependent dual effect of thrombin on impaired growth/apoptosis or mitogenesis in tumor cells. Blood. 2000; 95:3133–3138.20. Gordon KJ, Dong M, Chislock EM, Fields TA, Blobe GC. Loss of type III transforming growth factor beta receptor expression increases motility and invasiveness associated with epithelial to mesenchymal transition during pancreatic cancer progression. Carcinogenesis. 2008; 29:252–262.21. Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat Cell Biol. 2002; 4:487–494.22. Ren D, Minami Y, Nishita M. Critical role of Wnt5a-Ror2 signaling in motility and invasiveness of carcinoma cells following Snail-mediated epithelial-mesenchymal transition. Genes Cells. 2011; 16:304–315.23. Smadja DM, d'Audigier C, Weiswald LB, Badoual C, Dangles-Marie V, Mauge L, et al. The Wnt antagonist Dickkopf-1 increases endothelial progenitor cell angiogenic potential. Arterioscler Thromb Vasc Biol. 2010; 30:2544–2552.24. Gonzalez-Sancho JM, Aguilera O, Garcia JM, Pendas-Franco N, Pena C, Cal S, et al. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005; 24:1098–1103.25. Maehata T, Taniguchi H, Yamamoto H, Nosho K, Adachi Y, Miyamoto N, et al. Transcriptional silencing of Dickkopf gene family by CpG island hypermethylation in human gastrointestinal cancer. World J Gastroenterol. 2008; 14:2702–2714.26. Qin X, Zhang H, Zhou X, Wang C, Zhang X, Ye L. Proliferation and migration mediated by Dkk-1/Wnt/beta-catenin cascade in a model of hepatocellular carcinoma cells. Transl Res. 2007; 150:281–294.27. Sheng SL, Huang G, Yu B, Qin WX. Clinical significance and prognostic value of serum Dickkopf-1 concentrations in patients with lung cancer. Clin Chem. 2009; 55:1656–1664.28. Shi RY, Yang XR, Shen QJ, Yang LX, Xu Y, Qiu SJ, et al. High expression of Dickkopf-related protein 1 is related to lymphatic metastasis and indicates poor prognosis in intrahepatic cholangiocarcinoma patients after surgery. Cancer. 2013; 119:993–1003.29. Enomoto-Iwamoto M, Kitagaki J, Koyama E, Tamamura Y, Wu C, Kanatani N, et al. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol. 2002; 251:142–156.30. Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006; 11:1696–1701.31. Law AY, Wong CK. Stanniocalcin-2 promotes epithelial-mesenchymal transition and invasiveness in hypoxic human ovarian cancer cells. Exp Cell Res. 2010; 316:3425–3434.32. Chakravarty D, Roy SS, Babu CR, Dandamudi R, Curiel TJ, Vivas-Mejia P, et al. Therapeutic targeting of PELP1 prevents ovarian cancer growth and metastasis. Clin Cancer Res. 2011; 17:2250–2259.33. Merritt WM, Lin YG, Spannuth WA, Fletcher MS, Kamat AA, Han LY, et al. Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J Natl Cancer Inst. 2008; 100:359–372.34. Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell. 2008; 13:7–9.35. Bobek V, Kovarik J. Antitumor and antimetastatic effect of warfarin and heparins. Biomed Pharmacother. 2004; 58:213–219.36. Palma-Nicolas JP, Lopez-Colome AM. Thrombin induces slug-mediated E-cadherin transcriptional repression and the parallel up-regulation of N-cadherin by a transcription-independent mechanism in RPE cells. J Cell Physiol. 2013; 228:581–589.37. Chang LH, Chen CH, Huang DY, Pai HC, Pan SL, Teng CM. Thrombin induces expression of twist and cell motility via the hypoxia-inducible factor-1alpha translational pathway in colorectal cancer cells. J Cell Physiol. 2011; 226:1060–1068.38. Huang MT, Wei PL, Liu JJ, Liu DZ, Huey-Chun H, An J, et al. Knockdown of thrombomodulin enhances HCC cell migration through increase of ZEB1 and decrease of E-cadherin gene expression. Ann Surg Oncol. 2010; 17:3379–3385.39. Wang J, Jin L, Li X, Deng H, Chen Y, Lian Q, et al. Gossypol induces apoptosis in ovarian cancer cells through oxidative stress. Mol Biosyst. 2013; 9:1489–1497.40. Ma Y, Weimer J, Fredrik R, Adam-Klages S, Sebens S, Caliebe A, et al. Aurora kinase inhibitor AZD1152 has an additional effect of platinum on a sequential application at the human ovarian cancer cell line SKOV3. Arch Gynecol Obstet. 2013; 288:173–182.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Membrane Proteins Involved in Epithelial-Mesenchymal Transition and Tumor Invasion: Studies on TMPRSS4 and TM4SF5
- Consideration of EphA2 in relation to epithelial-mesenchymal transition in uterine endometrial cancer
- Targeting epithelial-mesenchymal transition pathway in hepatocellular carcinoma
- Role of the Epithelial-Mesenchymal Transition in Bladder Cancer: From Prognosis to Therapeutic Target
- Epithelial-Mesenchymal Transition in Head and Neck Cancer