J Gynecol Oncol.
2013 Jul;24(3):258-264. 10.3802/jgo.2013.24.3.258.
The combination of intravenous bevacizumab and metronomic oral cyclophosphamide is an effective regimen for platinum-resistant recurrent ovarian cancer
- Affiliations
-
- 1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Northwestern University Feinberg School of Medicine, Robert H Lurie Comprehensive Cancer Center, Chicago, IL, USA. nneubaue@nmff.org
- 2Department of Preventative Medicine, Northwestern University Feinberg School of Medicine, Robert H Lurie Comprehensive Cancer Center, Chicago, IL, USA.
- KMID: 2288533
- DOI: http://doi.org/10.3802/jgo.2013.24.3.258
Abstract
OBJECTIVE
To determine the efficacy, progression-free survival (PFS) and overall survival (OS) for the combination of intravenous bevacizumab and oral cyclophosphamide in heavily pretreated patients with recurrent ovarian carcinoma.
METHODS
A retrospective review was performed for all patients with recurrent ovarian carcinoma treated with intravenous bevacizumab 10 mg/kg every 14 days and oral cyclophosphamide 50 mg daily between January 2006 and December 2010. Response to treatment was determined by Response Evaluation Criteria in Solid Tumors criteria and/or CA-125 levels.
RESULTS
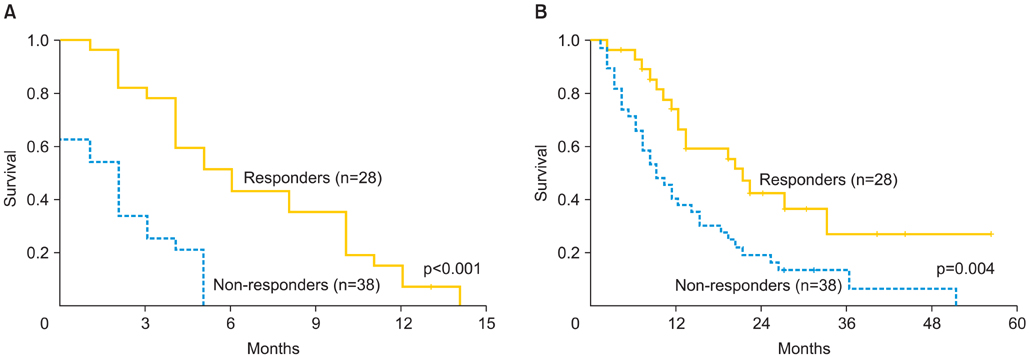
Sixty-six eligible patients were identified. Median age was 53 years. Fifty-five patients (83%) had undergone optimal cytoreduction. All patients were primarily or secondarily platinum resistant at the time of administration of bevacizumab and cyclophosphamide. The median number of prior chemotherapy treatments was 6.5 (range, 3 to 16). Eight patients (12.1%) had side effects which required discontinuation of bevacizumab and cyclophosphamide. There was one bowel perforation (1.5%). Overall response rate was 42.4%, including, complete response in 7 patients (10.6%), and partial response in 21 patients (31.8%), while 15 patients (22.7%) had stable disease and 23 patients (34.8%) had disease progression. Median PFS for responders was 5 months (range, 2 to 14 months). Median OS from initiation of bevacizumab and cyclophosphamide was 20 months (range, 2 to 56 months) for responders and 9 months (range, 2 to 51 months) for non-responders (p=0.004).
CONCLUSION
Bevacizumab and cyclophosphamide is an effective, well-tolerated chemotherapy regimen in heavily pretreated patients with recurrent ovarian carcinoma. This combination significantly improved PFS and OS in responders. Response rates were similar and favorable to the rates reported for similar patients receiving other commonly used second-line chemotherapeutic agents.
Keyword
MeSH Terms
Figure
Reference
-
1. National Cancer Institute. SEER stat fact sheets: ovary [Internet]. Bethesda, MD: National Cancer Institute;cited 2013 Feb 14. Available from: http://seer.cancer.gov/statfacts/html/ovary.html.2. Cannistra SA. Cancer of the ovary. N Engl J Med. 2004; 351:2519–2529.3. Abulafia O, Triest WE, Sherer DM. Angiogenesis in primary and metastatic epithelial ovarian carcinoma. Am J Obstet Gynecol. 1997; 177:541–547.4. Hartenbach EM, Olson TA, Goswitz JJ, Mohanraj D, Twiggs LB, Carson LF, et al. Vascular endothelial growth factor (VEGF) expression and survival in human epithelial ovarian carcinomas. Cancer Lett. 1997; 121:169–175.5. Paley PJ, Staskus KA, Gebhard K, Mohanraj D, Twiggs LB, Carson LF, et al. Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer. 1997; 80:98–106.6. Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007; 25:5165–5171.7. Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007; 25:5180–5186.8. Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001; 61:3369–3372.9. Jain RK. Antiangiogenic therapy for cancer: current and emerging concepts. Oncology (Williston Park). 2005; 19:7–16.10. Wildiers H, Guetens G, De Boeck G, Verbeken E, Landuyt B, Landuyt W, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. 2003; 88:1979–1986.11. Burger RA. Experience with bevacizumab in the management of epithelial ovarian cancer. J Clin Oncol. 2007; 25:2902–2908.12. Chura JC, Van Iseghem K, Downs LS Jr, Carson LF, Judson PL. Bevacizumab plus cyclophosphamide in heavily pretreated patients with recurrent ovarian cancer. Gynecol Oncol. 2007; 107:326–330.13. Matulonis UA, Pereira L, Liu J, Lee H, Lee J, Whalen C, et al. Sequential bevacizumab and oral cyclophosphamide for recurrent ovarian cancer. Gynecol Oncol. 2012; 126:41–46.14. Sanchez-Munoz A, Mendiola C, Perez-Ruiz E, Rodriguez-Sanchez CA, Jurado JM, Alonso-Carrion L, et al. Bevacizumab plus low-dose metronomic oral cyclophosphamide in heavily pretreated patients with recurrent ovarian cancer. Oncology. 2010; 79:98–104.15. Cheng X, Moroney JW, Levenback CF, Fu S, Jaishuen A, Kavanagh JJ. What is the benefit of bevacizumab combined with chemotherapy in patients with recurrent ovarian, fallopian tube or primary peritoneal malignancies? J Chemother. 2009; 21:566–572.16. Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008; 26:76–82.17. Alexandre J, Brown C, Coeffic D, Raban N, Pfisterer J, Maenpaa J, et al. CA-125 can be part of the tumour evaluation criteria in ovarian cancer trials: experience of the GCIG CALYPSO trial. Br J Cancer. 2012; 106:633–637.18. Herzog TJ, Vermorken JB, Pujade-Lauraine E, Provencher DM, Jagiello-Gruszfeld A, Kong B, et al. Correlation between CA-125 serum level and response by RECIST in a phase III recurrent ovarian cancer study. Gynecol Oncol. 2011; 122:350–355.19. Randall LM, Sill MW, Burger RA, Monk BJ, Buening B, Sorosky JI. Predictive value of serum CA-125 levels in patients with persistent or recurrent epithelial ovarian cancer or peritoneal cancer treated with bevacizumab on a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2012; 124:563–568.20. Jurado JM, Sanchez A, Pajares B, Perez E, Alonso L, Alba E. Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clin Transl Oncol. 2008; 10:583–586.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bevacizumab and oral metronomic cyclophosphamide in platinum-resistant ovarian cancer
- Improving Conventional or Low Dose Metronomic Chemotherapy with Targeted Antiangiogenic Drugs
- The role of topotecan as second-line chemotherapy in patients with recurrent epithelial ovarian cancer
- Two Cases of Advanced Bladder Carcinoma, Treated with CISCA Regimen
- Clinical Trial of IV Etoposide and Carboplatin and Cyclophosphamide Combination Chemotherapy Against Persistent or Recurrent Ovarian Cancer as 2nd or More Line Chemotherapy