J Clin Neurol.
2012 Dec;8(4):305-307. 10.3988/jcn.2012.8.4.305.
A Case of Lambert-Eaton Myasthenic Syndrome with Small-Cell Lung Cancer and Transient Increase in Anti-Acetylcholine-Receptor-Binding Antibody Titer
- Affiliations
-
- 1Department of Neurology, Yonsei University College of Medicine, Seoul, Korea. sunwooin@yumc.yonsei.ac.kr
- 2Department of Neurology, National Health Insurance Corporation Ilsan Hospital, Goyang, Korea.
- KMID: 2287583
- DOI: http://doi.org/10.3988/jcn.2012.8.4.305
Abstract
- BACKGROUND
Lambert-Eaton myasthenic syndrome (LEMS) is a presynaptic neuromuscular junction disorder that is most frequently associated with small-cell lung cancer (SCLC). The titers of antibodies against voltage-gated calcium channels are frequently increased in LEMS, but only rarely is titer of anti-acetylcholine-receptor-binding antibodies (AChR-abs) increased.
CASE REPORT
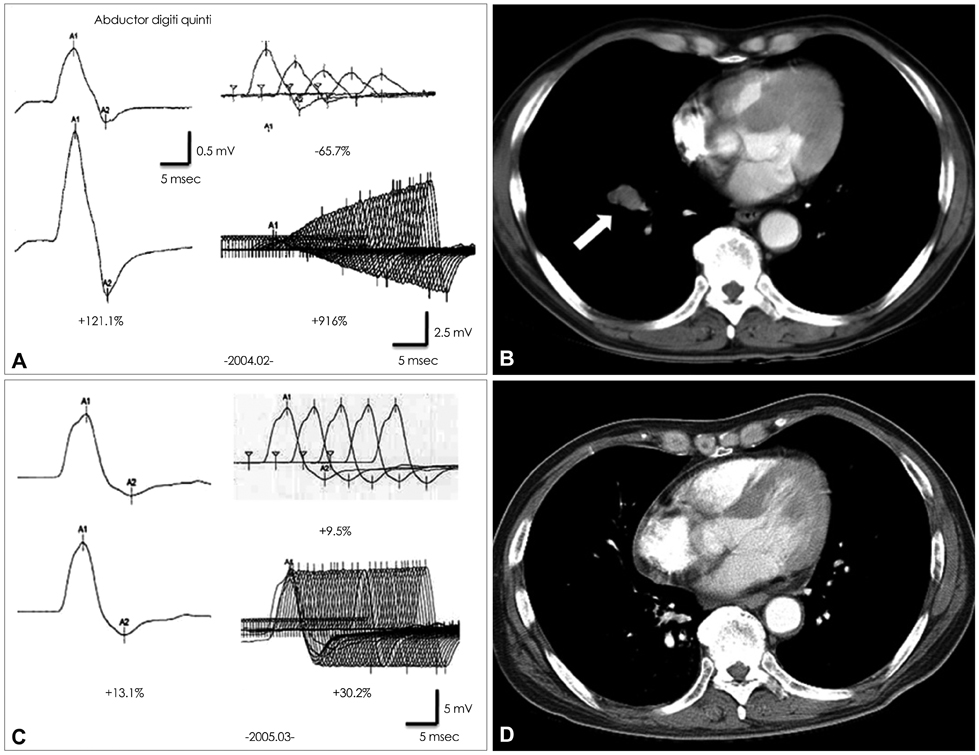
A 57-year-old male was admitted to our hospital due to dry mouth and eyes and progressive proximal limb weakness of 2 months duration. The results of a repetitive nerve stimulation test disclosed all criteria for the electrophysiological LEMS pattern, and the patient's AChR-abs titer was 0.587 nmol/L. At a follow-up performed 5 years after successful treatment of SCLC and LEMS, his AChR-abs titer had decreased to 0.001 nmol/L.
CONCLUSIONS
We suggest that this was a case of transient pseudopositivity of AChR-abs in SCLC with LEMS.
MeSH Terms
Figure
Reference
-
1. Harper CM Jr, Lennon VA. Kaminski HJ, editor. Lambert-Eaton syndrome. Myasthenia Gravis and Related Disorders. 2009. 2nd ed. New York: Humana Press;209–225.
Article2. Oh SJ. Principles of Clinical Electromyography, Case Studies. 1998. Baltimore: Williams & Wilkins;21–22.3. Lee SA, Sunwoo IN, Ro JK, Park KD. A case of remitted Lambert-Eaton myasthenic syndrome with small cell lung carcinoma following chemotherapy and radiotherapy. J Korean Neurol Assoc. 1992. 10:401–406.4. Katz JS, Wolfe GI, Bryan WW, Tintner R, Barohn RJ. Acetylcholine receptor antibodies in the Lambert-Eaton myasthenic syndrome. Neurology. 1998. 50:470–475.
Article5. Newsom-Davis J, Leys K, Vincent A, Ferguson I, Modi G, Mills K. Immunological evidence for the co-existence of the Lambert-Eaton myasthenic syndrome and myasthenia gravis in two patients. J Neurol Neurosurg Psychiatry. 1991. 54:452–453.
Article6. Taphoorn MJ, Van Duijn H, Wolters EC. A neuromuscular transmission disorder: combined myasthenia gravis and Lambert Eaton syndrome in one patient. J Neurol Neurosurg Psychiatry. 1988. 51:880–882.
Article7. Tabbaa MA, Leshner RT, Campbell WW. Malignant thymoma with dysautonomia and disordered neuromuscular transmission. Arch Neurol. 1986. 43:955–957.
Article8. Oh SJ, Sher E. MG and LEMS overlap syndrome: case report with electrophysiological and immunological evidence. Clin Neurophysiol. 2005. 116:1167–1171.
Article9. Fettel M, Shin H, Penn A, Lovelace R, Rowland L. Combined Eaton-Lambert syndrome and myasthenia gravis. Neurology. 1978. 28:398.10. Kaminski HJ, Santillan C, Wolfe GI. Autoantibody testing in neuromuscular disorders, part II: neuromuscular junction, hyperexcitability, and muscle disorders. J Clin Neuromuscul Dis. 2000. 2:96–105.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Marked Improvement of Lambert-Eaton Myasthenic Syndrome with the Chemotherapy of Small Cell Lung Carcinoma
- Seropositive Myasthenia Gravis Associated with Small-Cell Lung Carcinoma
- A Case of Lambert-Eaton Myasthenic Syndrome with Positive VGCC Antibodies Diagnosed in Small Cell Lung Cancer
- A Patient with Coexisting Myasthenia Gravis and Lambert-Eaton Myasthenic Syndrome
- A Case of Remitted Lambert-Eaton Myasthenic Syndrome with Small Cell Lung Carcinoma Following Chemotherapy and Radiotherapy