J Breast Cancer.
2010 Dec;13(4):337-348. 10.4048/jbc.2010.13.4.337.
Phosphorylated Akt and Phosphorylated mTOR Expression in Breast Invasive Carcinomas: Analysis of 530 Cases
- Affiliations
-
- 1Department of Pathology, Gil Medical Center, Gachon University of Medicine and Science, Incheon, Korea.
- 2Department of Pathology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea. ark@korea.ac.kr
- 3Forensic Medicine Division, Eastern District Office, National Institute of Scientific Investigation, Wonju, Korea.
- 4Department of Surgery, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- 5Division of Medical Oncology, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- KMID: 2286521
- DOI: http://doi.org/10.4048/jbc.2010.13.4.337
Abstract
- PURPOSE
The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway has a central role in regulation of cell proliferation, differentiation, motility and survival. This pathway has recently generated great interest because its elements are, potentially, novel targets for the treatment of various malignancies, including breast cancer.
METHODS
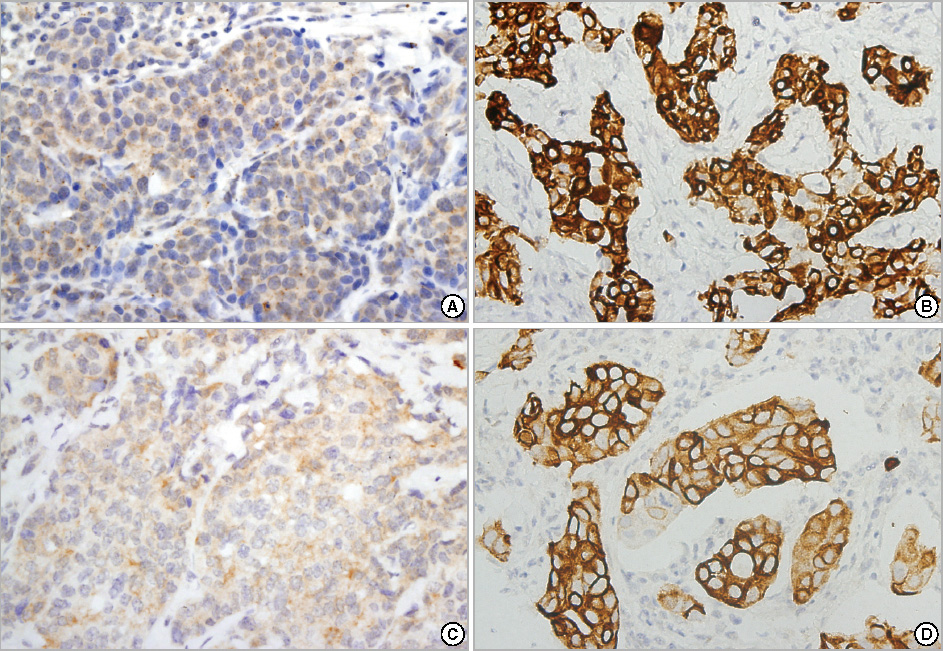
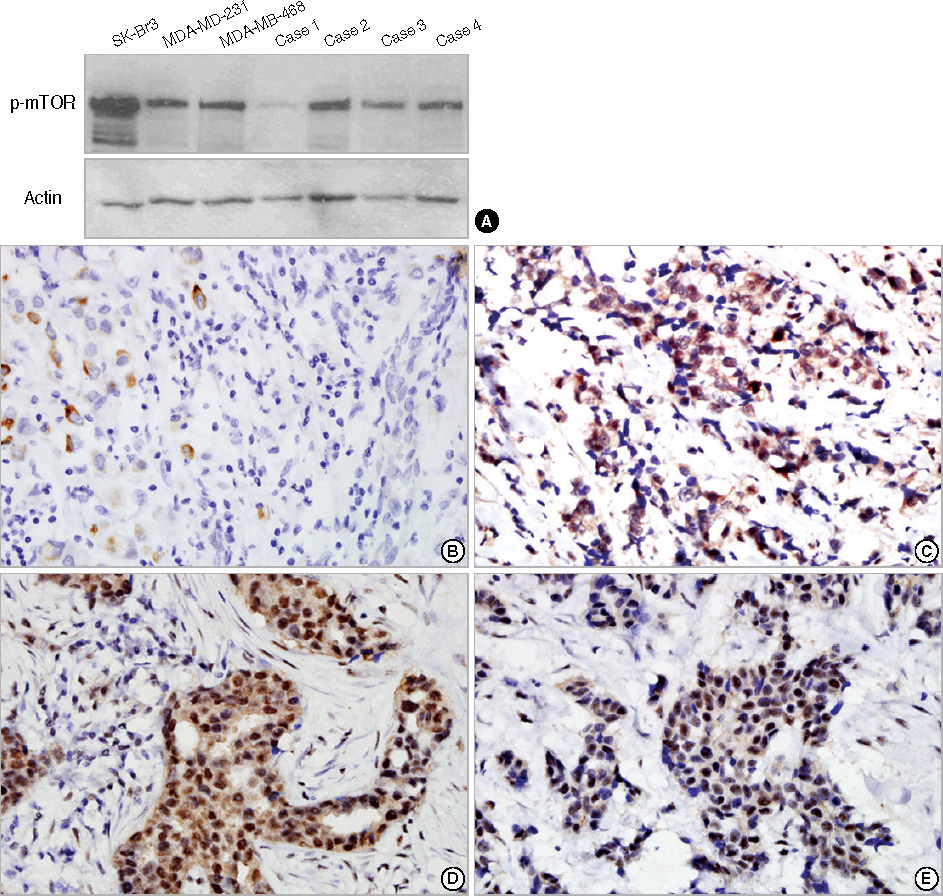
Using tissue microarray sections of breast carcinoma, we performed immunohistochemical studies using antibodies against the phosphorylated forms of Akt (p-Akt) and mTOR (p-mTOR) in 530 invasive breast carcinomas and 30 ductal carcinomas in situ (DCIS). We investigated possible associations between expression of these proteins and clinicopathologic characteristics and disease outcomes.
RESULTS
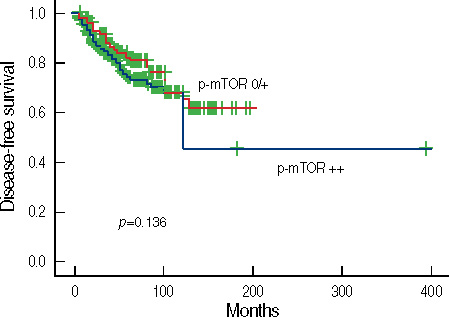
In 530 invasive carcinomas, weak and strong expression of p-Akt was observed in 180 (34.0%) and 288 (54.3%) cases, respectively. The expression of p-Akt was associated with expression of estrogen receptors (ER) (p=0.045), progesterone receptors (PR) (p=0.003), lymph node metastasis (p<0.001) and cancer stage (p=0.027). Weak and strong expression of p-mTOR was found in 136 (25.7%) and 207 (39.1%) cases, respectively. The mTOR pathway was more frequently activated in DCIS than in invasive breast carcinoma (p=0.001). p-mTOR expression was associated with expression of ER (p=0.040), PR (p=0.009), tumor size (p<0.001), and stage (p=0.002). In a univariate analysis, strong expression of p-Akt was associated with longer disease-free survival (DFS). In a multivariate analysis, neither p-Akt nor p-mTOR was associated with DFS.
CONCLUSION
The PI3K/Akt/mTOR pathway is active in DCIS as well as in invasive carcinoma of the breast. Our study also suggests that the PI3K/Akt/mTOR pathway is influenced by ER rather than erbB-2, and that this pathway may contribute more to cancer pathogenesis in ER-positive tumors.
Keyword
MeSH Terms
-
Antibodies
Breast
Carcinoma, Ductal
Carcinoma, Intraductal, Noninfiltrating
Cell Proliferation
Disease-Free Survival
Lymph Nodes
Multivariate Analysis
Neoplasm Metastasis
Phosphatidylinositol 3-Kinase
Proteins
Receptors, Estrogen
Receptors, Progesterone
Sirolimus
TOR Serine-Threonine Kinases
Antibodies
Phosphatidylinositol 3-Kinase
Proteins
Receptors, Estrogen
Receptors, Progesterone
Sirolimus
TOR Serine-Threonine Kinases
Figure
Reference
-
1. Borg A, Fernö M, Peterson C. Predicting the future of breast cancer. Nat Med. 2003. 9:16–18.
Article2. Lichtner RB. Estrogen/EGF receptor interactions in breast cancer: rationale for new therapeutic combination strategies. Biomed Pharmacother. 2003. 57:447–451.
Article3. Huang S, Houghton PJ. Targeting mTOR signaling for cancer therapy. Curr Opin Pharmacol. 2003. 3:371–377.
Article4. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006. 124:471–484.
Article5. Smolewski P. Recent developments in targeting the mammalian target of rapamycin (mTOR) kinase pathway. Anticancer Drugs. 2006. 17:487–494.
Article6. Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001. 15:807–826.
Article7. Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003. 4:117–126.
Article8. Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000. 103:253–262.
Article9. Zhou X, Tan M, Stone Hawthorne V, Klos KS, Lan KH, Yang Y, et al. Activation of the Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2 overexpression predicts tumor progression in breast cancers. Clin Cancer Res. 2004. 10:6779–6788.
Article10. Xu G, Zhang W, Bertram P, Zheng XF, McLeod H. Pharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR pathway in common human tumors. Int J Oncol. 2004. 24:893–900.
Article11. Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008. 26:4311–4318.
Article12. Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double blind, randomised, placebo-controlled phase III trial. Lancet. 2008. 372:449–456.
Article13. Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009. 27:2630–2637.
Article14. Baselga J. Combining the anti-EGFR agent gefitinib with chemotherapy in non-small-cell lung cancer: how do we go from INTACT to impact? J Clin Oncol. 2004. 22:759–761.
Article15. Elston CW, Ellis IO, Pinder SE. Pathological prognostic factors in breast cancer. Crit Rev Oncol Hematol. 1999. 31:209–223.
Article16. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999. 17:1474–1481.
Article17. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007. 25:118–145.
Article18. Pérez-Tenorio G, Stål O. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002. 86:540–545.
Article19. Panigrahi AR, Pinder SE, Chan SY, Paish EC, Robertson JF, Ellis IO. The role of PTEN and its signalling pathways, including AKT, in breast cancer; an assessment of relationships with other prognostic factors and with outcome. J Pathol. 2004. 204:93–100.
Article20. Bose S, Chandran S, Mirocha JM, Bose N. The Akt pathway in human breast cancer: a tissue-array-based analysis. Mod Pathol. 2006. 19:238–245.
Article21. Noh WC, Kim YH, Kim MS, Koh JS, Kim HA, Moon NM, et al. Activation of the mTOR signaling pathway in breast cancer and its correlation with the clinicopathologic variables. Breast Cancer Res Treat. 2008. 110:477–483.
Article22. Al-Bazz YO, Brown BL, Underwood JC, Stewart RL, Dobson PR. Immuno-analysis of phospho-Akt in primary human breast cancers. Int J Oncol. 2009. 35:1159–1167.
Article23. Anagnostou VK, Bepler G, Syrigos KN, Tanoue L, Gettinger S, Homer RJ, et al. High expression of mammalian target of rapamycin is associated with better outcome for patients with early stage lung adenocarcinoma. Clin Cancer Res. 2009. 15:4157–4164.
Article24. Sahin F, Kannangai R, Adegbola O, Wang J, Su G, Torbenson M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004. 10:8421–8425.
Article25. Klos KS, Wyszomierski SL, Sun M, Tan M, Zhou X, Li P, et al. ErbB2 increases vascular endothelial growth factor protein synthesis via activation of mammalian target of rapamycin/p70S6K leading to increased angiogenesis and spontaneous metastasis of human breast cancer cells. Cancer Res. 2006. 66:2028–2037.
Article26. Schmitz KJ, Otterbach F, Callies R, Levkau B, Hölscher H, Hoffmann O, et al. Prognostic relevance of activated Akt kinase in node-negative breast cancer: a clinicopathological study of 99 cases. Mod Pathol. 2004. 17:15–21.
Article27. Ellard SL, Clemons M, Gelmon KA, Norris B, Kennecke H, Chia S, et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol. 2009. 27:4536–4541.
Article28. Mori N, Kyo S, Sakaguchi J, Mizumoto Y, Ohno S, Maida Y, et al. Concomitant activation of AKT with extracellular-regulated kinase 1/2 occurs independently of PTEN or PIK3CA mutations in endometrial cancer and may be associated with favorable prognosis. Cancer Sci. 2007. 98:1881–1888.
Article29. Noh WC, Mondesire WH, Peng J, Jian W, Zhang H, Dong J, et al. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004. 10:1013–1023.
Article30. Cho D, Signoretti S, Dabora S, Regan M, Seeley A, Mariotti M, et al. Potential histologic and molecular predictors of response to temsirolimus in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2007. 5:379–385.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Analysis of Phosphorylated Akt Protein Expression in Gastric Carcinomas
- Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia
- Expression of Down Stream Molecules of RET (p-ERK, p-p38 MAPK, p-JNK and p-AKT) in Papillary Thyroid Carcinomas
- Increased Phosphorylation of PI3K/Akt/mTOR in the Obstructed Kidney of Rats with Unilateral Ureteral Obstruction
- Activation of the Renal PI3K/Akt/mTOR Signaling Pathway in a DOCA-Salt Model of Hypertension