J Breast Cancer.
2012 Mar;15(1):34-42. 10.4048/jbc.2012.15.1.34.
Circulating Tumor Cells Detected by RT-PCR for CK-20 before Surgery Indicate Worse Prognostic Impact in Triple-Negative and HER2 Subtype Breast Cancer
- Affiliations
-
- 1Division of Breast-Endocrine Surgery, Department of Surgery, Korea University Anam Hospital, Seoul, Korea. kujwbae@korea.ac.kr
- KMID: 2286471
- DOI: http://doi.org/10.4048/jbc.2012.15.1.34
Abstract
- PURPOSE
Circulating tumor cells (CTC) clearly correlate with unfavorable outcomes for patients with metastatic breast cancer, but the long-term prognostic implications of CTC for molecular subtypes of operable breast cancer are not yet known. We explored the relationships between previously established prognostic factors and CTC in operable breast cancer, and the significance of CTC by breast cancer molecular subtype.
METHODS
We retrospectively evaluated 166 patients with operable breast cancer (stage I-IIIA) diagnosed from April 1997 to May 2003. CTC were detected using cytokeratin-20 (CK-20) mRNA expression in peripheral blood samples that were collected just prior to surgery under general anesthesia. Clinicopathological characteristics of the cancer were analyzed according to CTC status. Metastasis-free survival (MFS) and overall survival (OS) were analyzed according to CTC status and breast cancer molecular subtype.
RESULTS
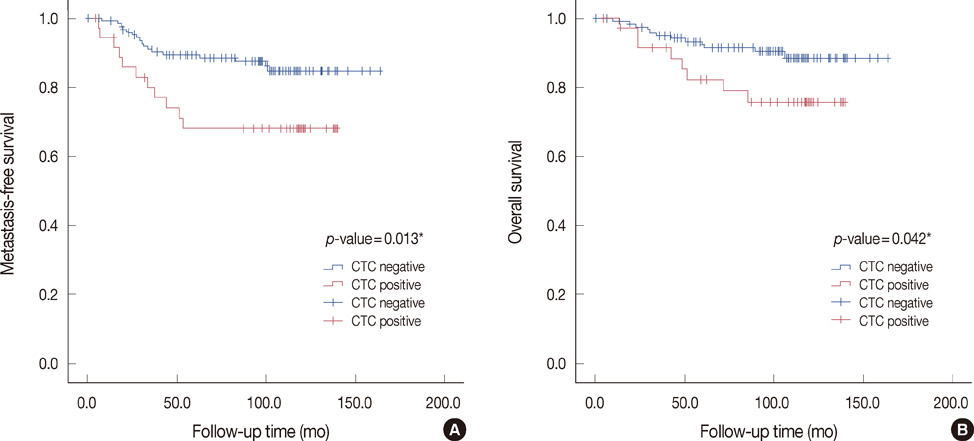
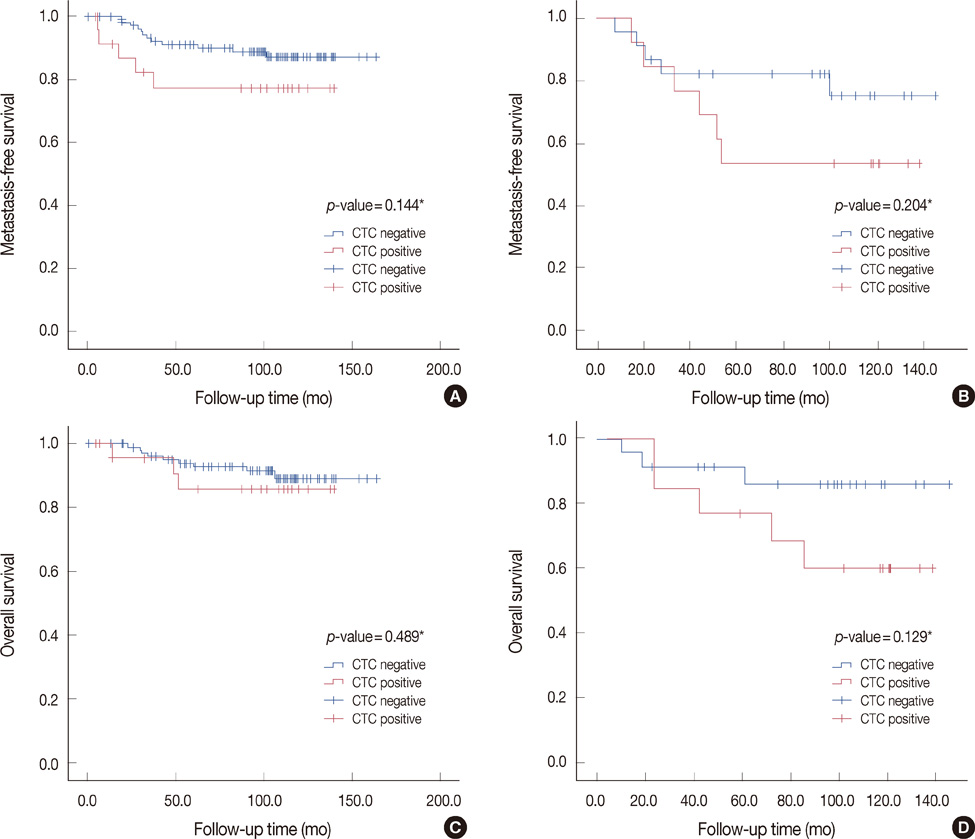
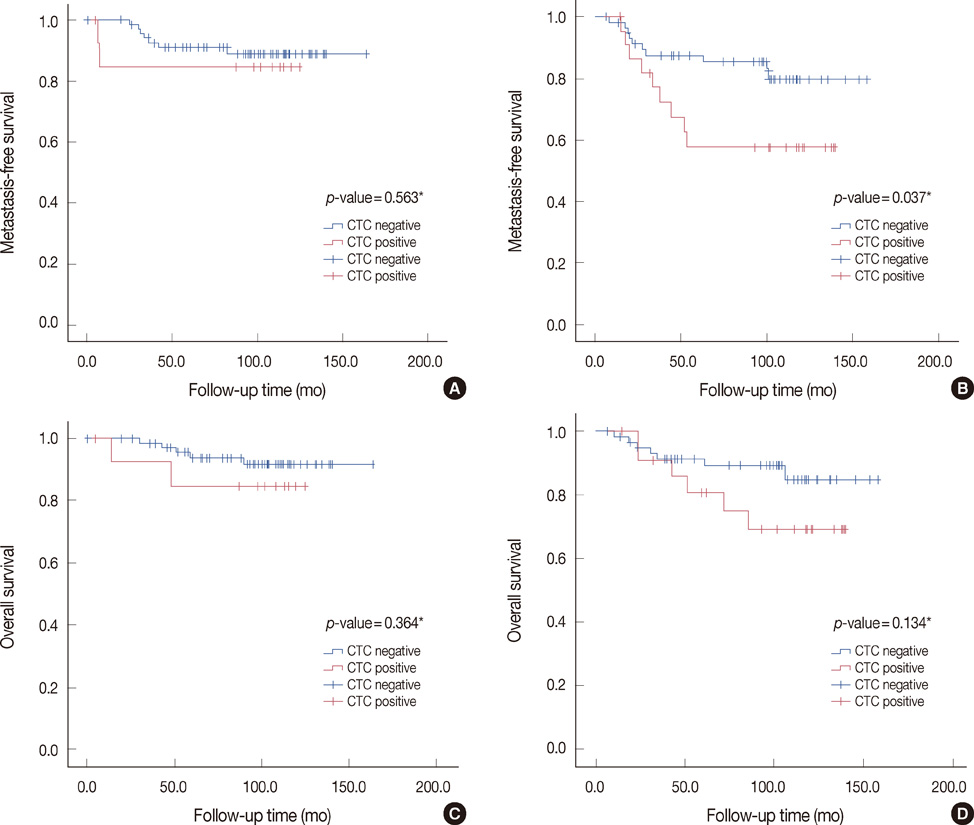
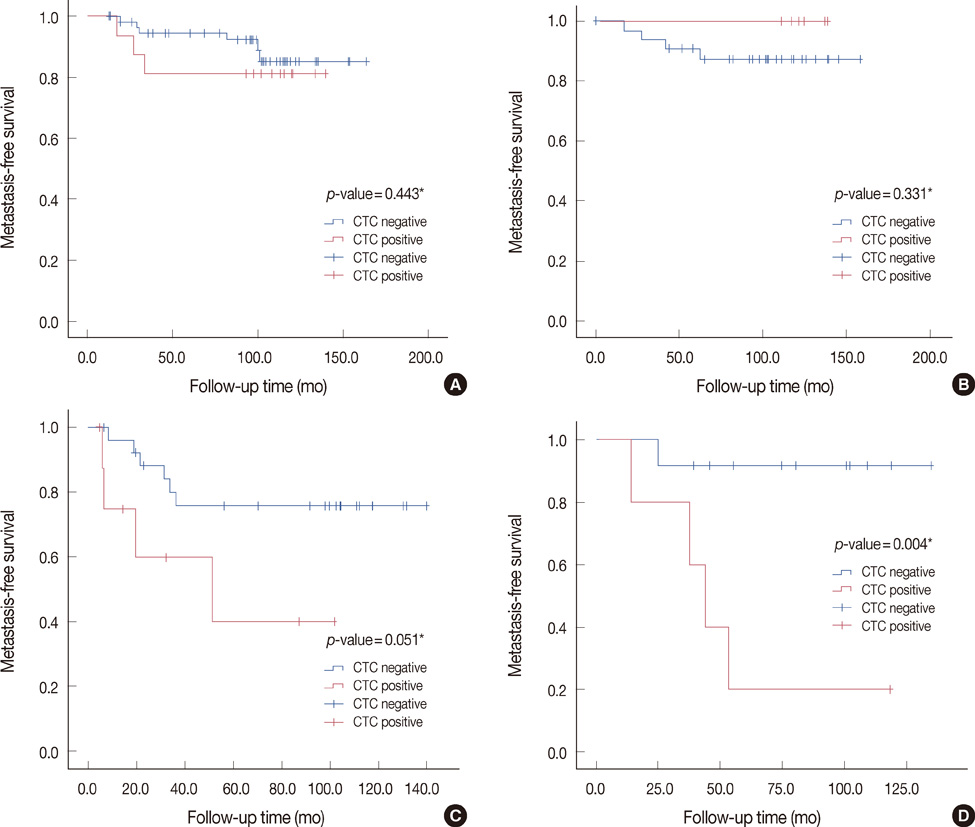
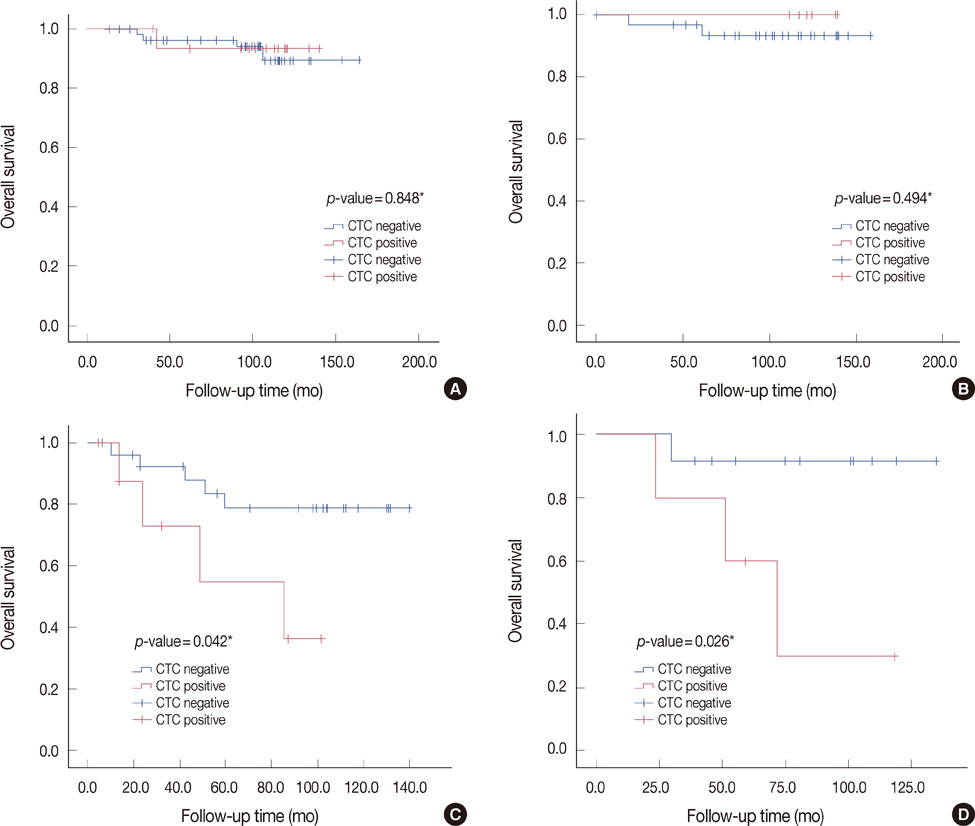
CK-20 mRNA-positive CTC was detected in 37 of 166 patients (22.3%) and was not correlated with any previous clinical factors in univariate analysis (p>0.05). After a median follow-up of 100 months, the patients with CK-20 mRNA-positive CTC had less favorable outcomes in terms of MFS and OS than those without detectable CTC (log-rank p<0.05). Among molecular subtypes of operable breast cancer, the patients with CK-20 mRNA-positive CTC had shorter MFS and OS in triple negative and human epidermal growth factor 2 (HER2) breast cancer subtype (log-rank, p<0.05).
CONCLUSION
CK-20 mRNA-positive CTC may lend insight into tumor progression as a prognostic indicator especially in the triple negative and HER2 subtypes of operable breast cancer.
MeSH Terms
Figure
Reference
-
1. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005. 365:1687–1717.2. Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008. 8:329–340.
Article3. Pantel K, Alix-Panabières C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009. 6:339–351.
Article4. Braun S, Pantel K, Müller P, Janni W, Hepp F, Kentenich CR, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med. 2000. 342:525–533.
Article5. Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005. 353:793–802.
Article6. Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004. 351:781–791.
Article7. Giuliano M, Giordano A, Jackson S, Hess KR, De Giorgi U, Mego M, et al. Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Res. 2011. 13:R67.
Article8. Daskalakis M, Mavroudis D, Sanidas E, Apostolaki S, Askoxylakis I, de Bree E, et al. Assessment of the effect of surgery on the kinetics of circulating tumour cells in patients with operable breast cancer based on cytokeratin-19 mRNA detection. Eur J Surg Oncol. 2011. 37:404–410.
Article9. Tao M, Ma D, Li Y, Zhou C, Zhang Y, Duan W, et al. Clinical significance of circulating tumor cells in breast cancer patients. Breast Cancer Res Treat. 2011. 129:247–254.
Article10. Graves H, Czerniecki BJ. Circulating tumor cells in breast cancer patients: an evolving role in patient prognosis and disease progression. Patholog Res Int. 2011. 2011:621090.
Article11. Bostick PJ, Chatterjee S, Chi DD, Huynh KT, Giuliano AE, Cote R, et al. Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol. 1998. 16:2632–2640.
Article12. Bae JW, Choi KH, Kim HG, Park SH. The detection of circulating breast cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction. J Korean Med Sci. 2000. 15:194–198.
Article13. Giribaldi G, Procida S, Ulliers D, Mannu F, Volpatto R, Mandili G, et al. Specific detection of cytokeratin 20-positive cells in blood of colorectal and breast cancer patients by a high sensitivity real-time reverse transcriptase-polymerase chain reaction method. J Mol Diagn. 2006. 8:105–112.
Article14. Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003. 100:8418–8423.
Article15. Kapp AV, Jeffrey SS, Langerød A, Børresen-Dale AL, Han W, Noh DY, et al. Discovery and validation of breast cancer subtypes. BMC Genomics. 2006. 7:231.
Article16. Normanno N, De Luca A, Carotenuto P, Lamura L, Oliva I, D'Alessio A. Prognostic applications of gene expression signatures in breast cancer. Oncology. 2009. 77:Suppl 1. 2–8.
Article17. Ignatiadis M, Xenidis N, Perraki M, Apostolaki S, Politaki E, Kafousi M, et al. Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol. 2007. 25:5194–5202.
Article18. Riethdorf S, Pantel K. Disseminated tumor cells in bone marrow and circulating tumor cells in blood of breast cancer patients: current state of detection and characterization. Pathobiology. 2008. 75:140–148.
Article19. Kim J, Bae JW, Lee JB, Son GS, Koo BH. RT-PCR amplification of CK 20 mRNA in the peripheral blood of breast cancer patients: correlation with established prognostic parameters. Biomed Pharmacother. 2005. 59:Suppl 2. S380–S383.
Article20. Lang JE, Mosalpuria K, Cristofanilli M, Krishnamurthy S, Reuben J, Singh B, et al. HER2 status predicts the presence of circulating tumor cells in patients with operable breast cancer. Breast Cancer Res Treat. 2009. 113:501–507.
Article21. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005. 353:1659–1672.22. Dawood S. Triple-negative breast cancer: epidemiology and management options. Drugs. 2010. 70:2247–2258.23. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005. 353:1673–1684.
Article24. Gaedcke J, Traub F, Milde S, Wilkens L, Stan A, Ostertag H, et al. Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod Pathol. 2007. 20:864–870.
Article25. Ignatiadis M, Rothé F, Chaboteaux C, Durbecq V, Rouas G, Criscitiello C, et al. HER2-positive circulating tumor cells in breast cancer. PLoS One. 2011. 6:e15624.
Article26. Pestrin M, Bessi S, Galardi F, Truglia M, Biggeri A, Biagioni C, et al. Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat. 2009. 118:523–530.
Article27. Kim SI, Jung H. Circulating tumor cells: detection methods and potential clinical application in breast cancer. J Breast Cancer. 2010. 13:125–131.
Article28. Riethdorf S, Pantel K. Advancing personalized cancer therapy by detection and characterization of circulating carcinoma cells. Ann N Y Acad Sci. 2010. 1210:66–77.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Influence of BCL2 on Molecular Subtypes of Breast Cancer
- Biologic subtype is a more important prognostic factor than nodal involvement in patients with stages I and II breast carcinoma
- Different Prognostic Significance of Bcl-2 Based on Cancer Molecular Subtype
- The detection of circulating breast cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction
- Prognostic Value of the Evolution of HER2-Low Expression after Neoadjuvant Chemotherapy