J Breast Cancer.
2012 Sep;15(3):288-295. 10.4048/jbc.2012.15.3.288.
Survival Benefit of Tamoxifen in Estrogen Receptor-Negative and Progesterone Receptor-Positive Low Grade Breast Cancer Patients
- Affiliations
-
- 1Department of Surgery, Changhua Christian Hospital, Changhua, Taiwan. darren_chen@cch.org.tw
- 2Department of Medical Research, Changhua Christian Hospital, Changhua, Taiwan.
- 3Centre of Biostatistics Consultation, National Taiwan University College of Public Health, Taipei, Taiwan.
- 4School of Oral Hygiene, Taipei Medical University College of Oral Medicine, Taipei, Taiwan.
- 5Comprehensive Breast Cancer Center, Changhua Christian Hospital, Changhua, Taiwan.
- KMID: 2286454
- DOI: http://doi.org/10.4048/jbc.2012.15.3.288
Abstract
- PURPOSE
This study aimed to analyze the efficacy and prognostic significance of adjuvant tamoxifen in breast cancer patients with various hormone receptor statuses.
METHODS
Typically, 1,260 female breast cancer patients were recruited in this study. The correlation between estrogen receptor (ER)/progesterone receptor (PR) phenotypes and clinical characteristics was investigated, and the survival rate was assessed after 5-year follow-up.
RESULTS
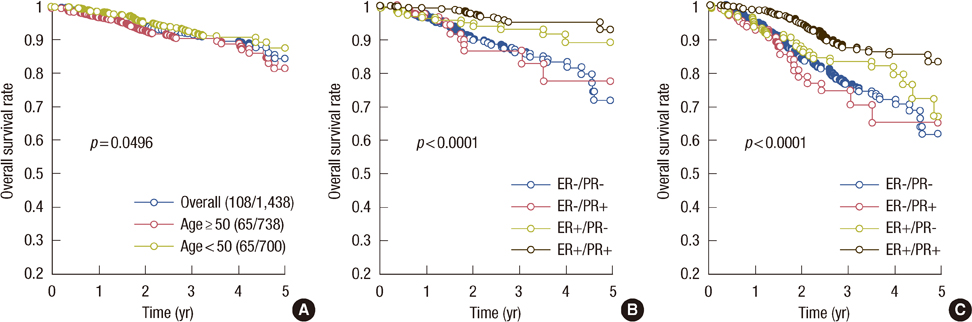
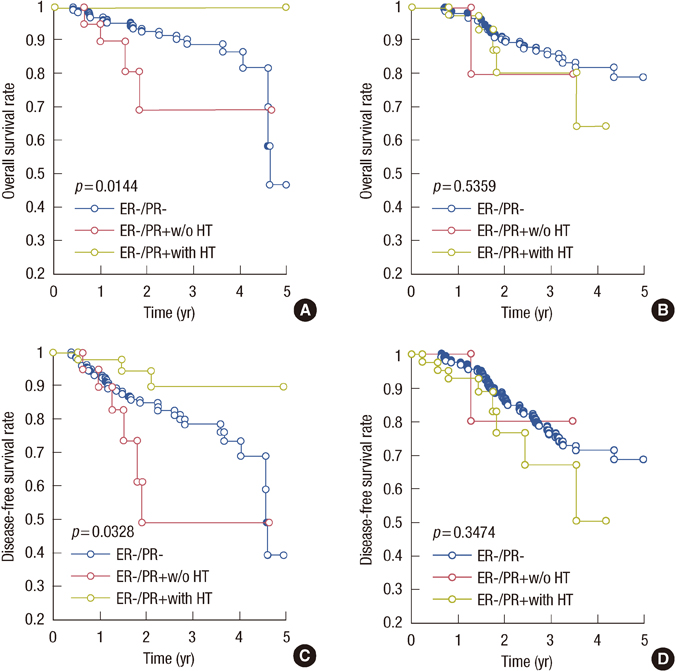
The 5-year overall survival (85%) was better in women under the age of 50 years. Patients with ER+/PR+ tumors had a better 5-year survival rate (94%); those with ER-/PR- tumors experienced the worst outcome (74% survival rate); whereas single-positive cases were in between. In 97 out of 128 patients with ER-/PR+ tumors, tamoxifen was given as adjuvant hormonal therapy, and it increased the survival benefit in the lower grade group in terms of overall survival and disease-free survival (p=0.01 and p=0.03, respectively).
CONCLUSION
For high-grade tumors with ER-/PR+, adjuvant tamoxifen therapy may have no survival benefit, whereas for the patients with low-grade ER-/PR+ tumors, adjuvant tamoxifen therapy is highly suggestive.
MeSH Terms
Figure
Cited by 1 articles
-
If Progesterone Is Blamed for Breast Cancer Development, Why Are We Still Using Tamoxifen?
Enis Özkaya, Vakkas Korkmaz, Tuncay Kucukozkan, Fadil Kara
J Breast Cancer. 2013;16(1):131-131. doi: 10.4048/jbc.2013.16.1.131.
Reference
-
1. Fisher B, Anderson S, Tan-Chiu E, Wolmark N, Wickerham DL, Fisher ER, et al. Tamoxifen and chemotherapy for axillary node-negative, estrogen receptor-negative breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-23. J Clin Oncol. 2001. 19:931–942.
Article2. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005. 365:1687–1717.3. Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998. 351:1451–1467.4. Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005. 365:60–62.
Article5. Osborne CK, Schiff R, Arpino G, Lee AS, Hilsenbeck VG. Endocrine responsiveness: understanding how progesterone receptor can be used to select endocrine therapy. Breast. 2005. 14:458–465.
Article6. Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, et al. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol. 2007. 25:4772–4778.
Article7. Anderson WF, Chen BE, Jatoi I, Rosenberg PS. Effects of estrogen receptor expression and histopathology on annual hazard rates of death from breast cancer. Breast Cancer Res Treat. 2006. 100:121–126.
Article8. Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005. 97:1254–1261.
Article9. Olivotto IA, Truong PT, Speers CH, Bernstein V, Allan SJ, Kelly SJ, et al. Time to stop progesterone receptor testing in breast cancer management. J Clin Oncol. 2004. 22:1769–1770.
Article10. Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003. 21:1973–1979.
Article11. Greene FL. American Joint Committee on Cancer, American Cancer Society. AJCC Cancer Staging Manual. 2002. 6th ed. Philadelphia: Springer.12. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006. 295:2492–2502.
Article13. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: breast cancer- v.1. 2012. Accessed March 8th, 2012. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.14. Osborne CK, Yochmowitz MG, Knight WA 3rd, McGuire WL. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer. 1980. 46:12 Suppl. 2884–2888.
Article15. Loprinzi CL, Ravdin PM, de Laurentiis M, Novotny P. Do American oncologists know how to use prognostic variables for patients with newly diagnosed primary breast cancer? J Clin Oncol. 1994. 12:1422–1426.
Article16. Breast International Group (BIG) 1-98 Collaborative Group. Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005. 353:2747–2757.
Article17. Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005. 366:455–462.
Article18. Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004. 350:1081–1092.
Article19. Kaufmann M, Jonat W, Hilfrich J, Eidtmann H, Gademann G, Zuna I, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol. 2007. 25:2664–2670.
Article20. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005. 97:1262–1271.
Article21. Bird PA, Hill AG, Houssami N. Poor hormone receptor expression in East African breast cancer: evidence of a biologically different disease? Ann Surg Oncol. 2008. 15:1983–1988.
Article22. Yu KD, Di GH, Wu J, Lu JS, Shen KW, Liu GY, et al. Breast cancer patients with estrogen receptor-negative/progesterone receptor-positive tumors: being younger and getting less benefit from adjuvant tamoxifen treatment. J Cancer Res Clin Oncol. 2008. 134:1347–1354.
Article23. Nikolic-Vukosavljevic D, Kanjer K, Neskovic-Konstantinovic Z, Vukotic D. Natural history of estrogen receptor-negative, progesterone receptor-positive breast cancer. Int J Biol Markers. 2002. 17:196–200.
Article24. Mc Cormack O, Harrison M, Kerin MJ, McCann A. Role of the progesterone receptor (PR) and the PR isoforms in breast cancer. Crit Rev Oncog. 2007. 13:283–301.
Article25. Rakha EA, El-Sayed ME, Lee AH, Elston CW, Grainge MJ, Hodi Z, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008. 26:3153–3158.
Article26. Piccart MJ, Di Leo A, Hamilton A. HER2: a 'predictive factor' ready to use in the daily management of breast cancer patients? Eur J Cancer. 2000. 36:1755–1761.27. Berry DA, Muss HB, Thor AD, Dressler L, Liu ET, Broadwater G, et al. HER-2/neu and p53 expression versus tamoxifen resistance in estrogen receptor-positive, node-positive breast cancer. J Clin Oncol. 2000. 18:3471–3479.
Article28. De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005. 11:4741–4748.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Tamoxifen of the Estrogen Receptor cDNA-Iipofected MDA-MB-231 Human Breast Cancer Cells
- Treatment Outcomes of Weakly Positive Hormone Receptor Breast Cancer and Triple-Negative Breast Cancer
- Restoration of Hormone Dependency in Estrogen Receptor - Lipofected MDA-MB-231 Human Breast Cancer Cells
- The Clinical Significance of the Estrogen Receptor beta Expression for Endocrine Therapy in Patients with ERalpha-negative and Progesterone Receptor-positive Breast Carcinoma
- Effects of Tamoxifen on Bone Mineral Metabolism in Women with Breast Cancer