J Breast Cancer.
2012 Sep;15(3):273-282. 10.4048/jbc.2012.15.3.273.
Stem Cell Implants for Cancer Therapy: TRAIL-Expressing Mesenchymal Stem Cells Target Cancer Cells In Situ
- Affiliations
-
- 1Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, USA.
- 2Department of Medicine, Harvard Medical School, Boston, USA.
- 3Department of Biomedical Engineering, Tufts University, Medford, USA. David.kaplan@tufts.edu
- 4Centre for Respiratory Research, University College London, London, UK.
- KMID: 2286452
- DOI: http://doi.org/10.4048/jbc.2012.15.3.273
Abstract
- PURPOSE
Tumor-specific delivery of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), an apoptosis-inducing peptide, at effective doses remains challenging. Herein we demonstrate the utility of a scaffold-based delivery system for sustained therapeutic cell release that capitalizes on the tumor-homing properties of mesenchymal stem cells (MSCs) and their ability to express genetically-introduced therapeutic genes.
METHODS
Implants were formed from porous, biocompatible silk scaffolds seeded with full length TRAIL-expressing MSCs (FLT-MSCs). under a doxycycline inducible promoter. In vitro studies with FLT-MSCs demonstrated TRAIL expression and antitumor effects on breast cancer cells. Next, FLT-MSCs were administered to mice using three administration routes (mammary fat pad co-injections, tail vein injections, and subcutaneous implantation on scaffolds).
RESULTS
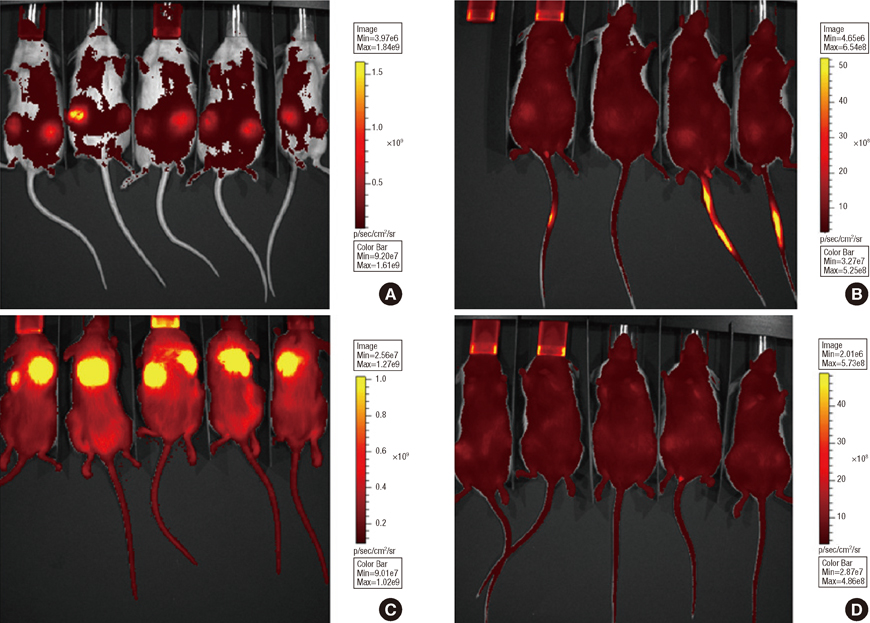
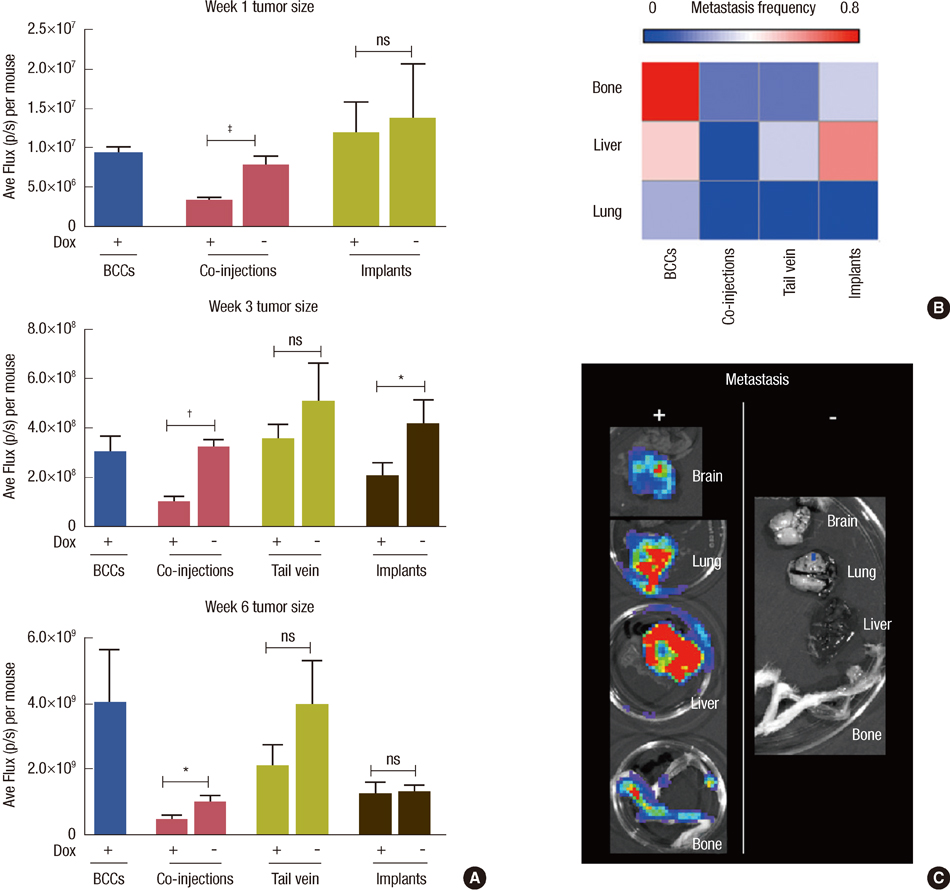
In vitro cell-specific bioluminescent imaging measured tumor cell specific growth in the presence of stromal cells and demonstrated FLT-MSC inhibition of breast cancer growth. FLT-MSC implants successfully decreased bone and lung metastasis, whereas liver metastasis decreased only with tail vein and co-injection administration routes. Average tumor burden was decreased when doxycycline was used to induce TRAIL expression for co-injection and scaffold groups, as compared to controls with no induced TRAIL expression.
CONCLUSION
This implant-based therapeutic delivery system is an effective and completely novel method of anticancer therapy and holds great potential for clinical applications.
Keyword
MeSH Terms
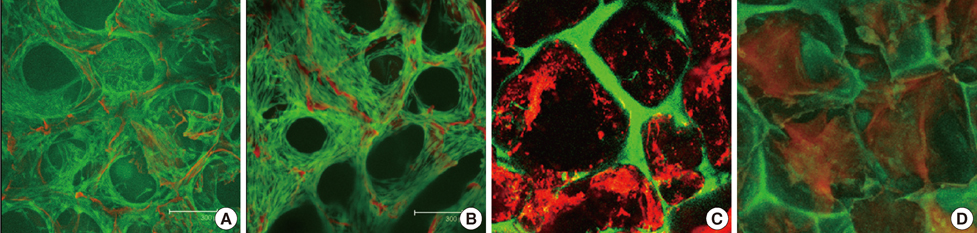
Figure
Reference
-
1. Lee JH, Yim SH, Won YJ, Jung KW, Son BH, Lee HD, et al. Population-based breast cancer statistics in Korea during 1993-2002: incidence, mortality, and survival. J Korean Med Sci. 2007. 22:Suppl. S11–S16.
Article2. Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 2010. 70:10044–10050.
Article3. Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007. 449:557–563.
Article4. Reagan MR, Kaplan DL. Concise review. Mesenchymal stem cell tumor-homing: detection methods in disease model systems. Stem Cells. 2011. 29:920–927.
Article5. Loebinger MR, Kyrtatos PG, Turmaine M, Price AN, Pankhurst Q, Lythgoe MF, et al. Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res. 2009. 69:8862–8867.
Article6. Liu Y, Lang F, Xie X, Prabhu S, Xu J, Sampath D, et al. Efficacy of adenovirally expressed soluble TRAIL in human glioma organotypic slice culture and glioma xenografts. Cell Death Dis. 2011. 2:e121.
Article7. El-Deiry WS. Death Receptors in Cancer Therapy. 2005. Totowa: Humana Press;374.8. Kagawa S, He C, Gu J, Koch P, Rha SJ, Roth JA, et al. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001. 61:3330–3338.9. Müller N, Schneider B, Pfizenmaier K, Wajant H. Superior serum half life of albumin tagged TNF ligands. Biochem Biophys Res Commun. 2010. 396:793–799.
Article10. Dörr J, Bechmann I, Waiczies S, Aktas O, Walczak H, Krammer PH, et al. Lack of tumor necrosis factor-related apoptosis-inducing ligand but presence of its receptors in the human brain. J Neurosci. 2002. 22:RC209.
Article11. Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000. 6:564–567.
Article12. Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS, et al. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008. 29:3415–3428.
Article13. Zhao X, Kim J, Cezar CA, Huebsch N, Lee K, Bouhadir K, et al. Active scaffolds for on-demand drug and cell delivery. Proc Natl Acad Sci USA. 2011. 108:67–72.
Article14. Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009. 8:151–158.
Article15. Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009. 69:4134–4142.
Article16. Moreau JE, Anderson K, Mauney JR, Nguyen T, Kaplan DL, Rosenblatt M. Tissue-engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res. 2007. 67:10304–10308.
Article17. McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010. 16:483–489.
Article18. Bhardwaj N, Nguyen QT, Chen AC, Kaplan DL, Sah RL, Kundu SC. Potential of 3-D tissue constructs engineered from bovine chondrocytes/silk fibroin-chitosan for in vitro cartilage tissue engineering. Biomaterials. 2011. 32:5773–5781.
Article19. Lü K, Xu L, Xia L, Zhang Y, Zhang X, Kaplan DL, et al. An ectopic study of apatite-coated silk fibroin scaffolds seeded with AdBMP-2-modified canine bMSCs. J Biomater Sci Polym Ed. 2012. 23:509–526.
Article20. Wang H, Cao F, De A, Cao Y, Contag C, Gambhir SS, et al. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells. 2009. 27:1548–1558.
Article21. Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005. 5:591–602.
Article22. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009. 5:54–63.
Article23. Roodhart JM, Daenen LG, Stigter EC, Prins HJ, Gerrits J, Houthuijzen JM, et al. Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell. 2011. 20:370–383.
Article24. Soria JC, Smit E, Khayat D, Besse B, Yang X, Hsu CP, et al. Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non-small-cell lung cancer. J Clin Oncol. 2010. 28:1527–1533.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells
- Cancer Stem Cells in Brain Tumors and Their Lineage Hierarchy
- Role of Gastric Stem Cells in Gastric Carcinogenesis by Chronic Helicobacter pylori Infection
- New Findings on Breast Cancer Stem Cells: A Review
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease