J Breast Cancer.
2014 Sep;17(3):200-206. 10.4048/jbc.2014.17.3.200.
siRNA-Mediated Suppression of Synuclein gamma Inhibits MDA-MB-231 Cell Migration and Proliferation by Downregulating the Phosphorylation of AKT and ERK
- Affiliations
-
- 1Department of Breast Surgery, The First Affiliated Hospital of Shenzhen University, Second People's Hospital of Shen Zhen, Shen Zhen, China. jinsonghe116@163.com
- 2Biobank, The First Affiliated Hospital of Shenzhen University, Second People's Hospital of Shen Zhen, Shen Zhen, China.
- 3Department of Laboratory Medicine and Pathology, Masonic Cancer Center, University of Minnesota, Minneapolis, MN, USA.
- 4Department of Pathology, The First Affiliated Hospital of Shenzhen University, Second People's Hospital of Shen Zhen, Shen Zhen, China.
- KMID: 2286346
- DOI: http://doi.org/10.4048/jbc.2014.17.3.200
Abstract
- PURPOSE
Synuclein-gamma (SNCG), which was initially identified as breast cancer specific gene 1, is highly expressed in advanced breast cancers, but not in normal or benign breast tissue. This study aimed to evaluate the effects of SNCG siRNA-treatment on breast cancer cells and elucidate the associated mechanisms.
METHODS
Vectors containing SNCG and negative control (NC) siRNAs were transfected into MDA-MB-231 cells; mRNA levels were determined by real-time polymerase chain reaction. Cell proliferation was evaluated using the MTT assay, cell migration was assessed by the Transwell assay, apoptosis and cell cycle analyses were conducted with the flow cytometer, and Western blot analysis was performed to determine the relative levels of AKT, ERK, p-AKT, and p-ERK expression.
RESULTS
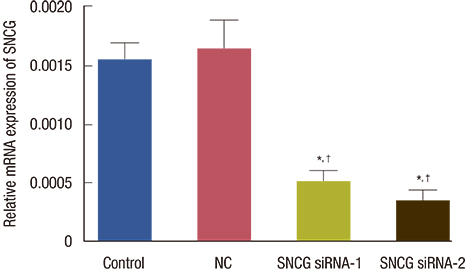
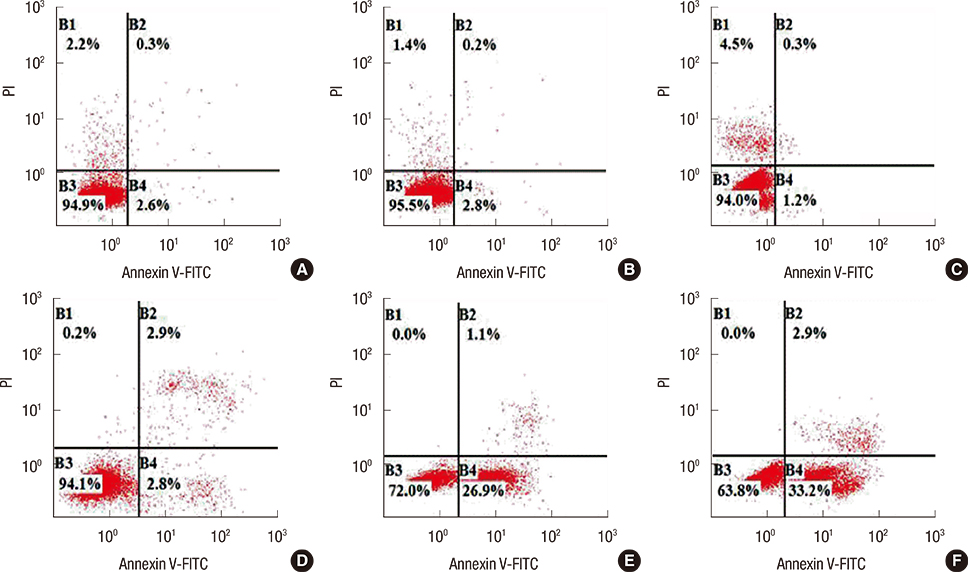
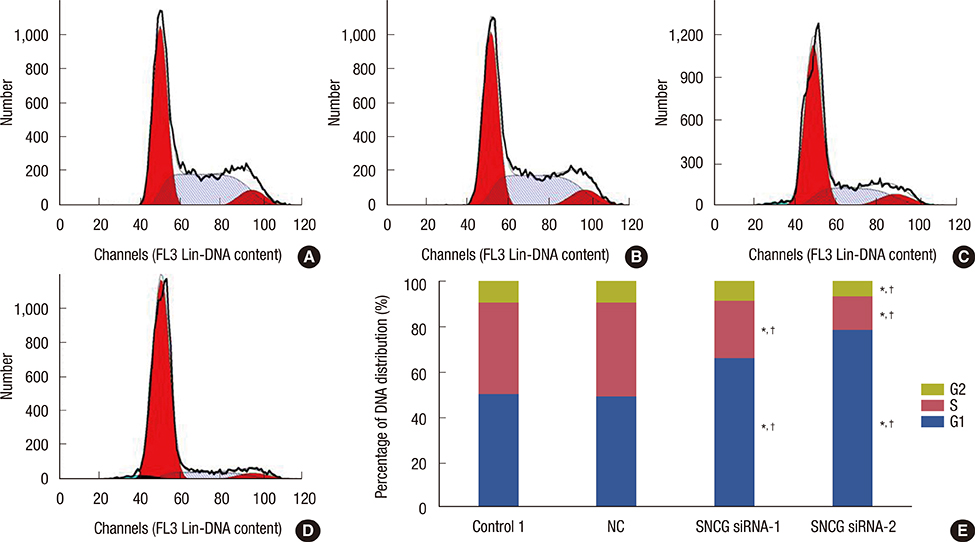
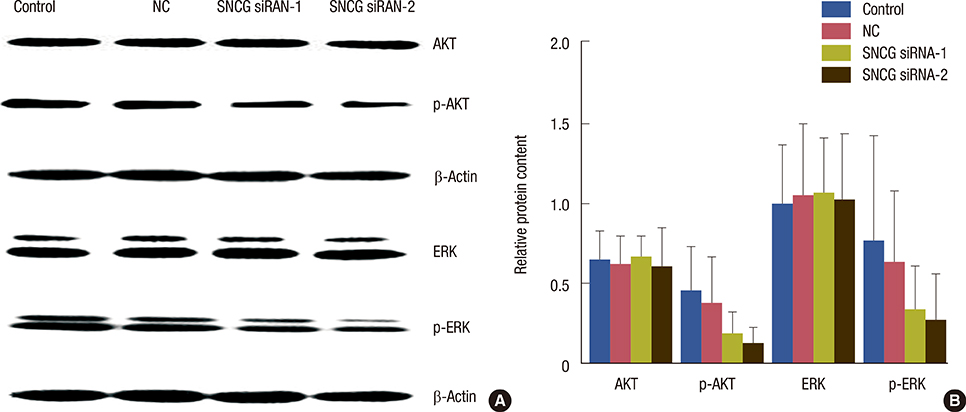
SNCG mRNA levels were significantly reduced in MDA-MB-231 cells transfected with SNCG siRNA. Our results indicate that in SNCG siRNA-treated cells, cell migration and proliferation decreased significantly, apoptosis was induced, and the cell cycle was arrested. Western blot analysis indicated that the protein levels of p-AKT and p-ERK were much lower in the SNCG siRNA-treated groups, than in the control and NC groups.
CONCLUSION
SNCG siRNA could decrease the migration and proliferation of breast cancer cells by downregulating the phosphorylation of AKT and ERK.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
Breast
Breast Neoplasms
Cell Cycle
Cell Migration Assays
Cell Movement*
Cell Proliferation
Extracellular Signal-Regulated MAP Kinases
Phosphorylation*
Proto-Oncogene Proteins c-akt
Real-Time Polymerase Chain Reaction
RNA, Messenger
RNA, Small Interfering
Synucleins*
Extracellular Signal-Regulated MAP Kinases
Proto-Oncogene Proteins c-akt
RNA, Messenger
RNA, Small Interfering
Synucleins
Figure
Reference
-
1. Smolarek AK, Suh N. Chemopreventive activity of vitamin E in breast cancer: a focus on γ- and δ-tocopherol. Nutrients. 2011; 3:962–986.
Article2. Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998; 21:249–254.
Article3. Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997; 276:2045–2047.
Article4. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997; 388:839–840.5. Ji H, Liu YE, Jia T, Wang M, Liu J, Xiao G, et al. Identification of a breast cancer-specific gene, BCSG1, by direct differential cDNA sequencing. Cancer Res. 1997; 57:759–764.6. Jia T, Liu YE, Liu J, Shi YE. Stimulation of breast cancer invasion and metastasis by synuclein gamma. Cancer Res. 1999; 59:742–747.7. Bruening W, Giasson BI, Klein-Szanto AJ, Lee VM, Trojanowski JQ, Godwin AK. Synucleins are expressed in the majority of breast and ovarian carcinomas and in preneoplastic lesions of the ovary. Cancer. 2000; 88:2154–2163.
Article8. Wu K, Weng Z, Tao Q, Lin G, Wu X, Qian H, et al. Stage-specific expression of breast cancer-specific gene gamma-synuclein. Cancer Epidemiol Biomarkers Prev. 2003; 12:920–925.9. Liu H, Liu W, Wu Y, Zhou Y, Xue R, Luo C, et al. Loss of epigenetic control of synuclein-gamma gene as a molecular indicator of metastasis in a wide range of human cancers. Cancer Res. 2005; 65:7635–7643.
Article10. Guo J, Shou C, Meng L, Jiang B, Dong B, Yao L, et al. Neuronal protein synuclein gamma predicts poor clinical outcome in breast cancer. Int J Cancer. 2007; 121:1296–1305.
Article11. Jiang Y, Liu YE, Goldberg ID, Shi YE. Gamma synuclein, a novel heat-shock protein-associated chaperone, stimulates ligand-dependent estrogen receptor alpha signaling and mammary tumorigenesis. Cancer Res. 2004; 64:4539–4546.
Article12. Zhou Y, Inaba S, Liu J. Inhibition of synuclein-gamma expression increases the sensitivity of breast cancer cells to paclitaxel treatment. Int J Oncol. 2006; 29:289–295.13. Pan ZZ, Bruening W, Giasson BI, Lee VM, Godwin AK. Gamma-synuclein promotes cancer cell survival and inhibits stress- and chemotherapy drug-induced apoptosis by modulating MAPK pathways. J Biol Chem. 2002; 277:35050–35060.
Article14. Singh VK, Zhou Y, Marsh JA, Uversky VN, Forman-Kay JD, Liu J, et al. Synuclein-gamma targeting peptide inhibitor that enhances sensitivity of breast cancer cells to antimicrotubule drugs. Cancer Res. 2007; 67:626–633.
Article15. Lavedan C, Leroy E, Dehejia A, Buchholtz S, Dutra A, Nussbaum RL, et al. Identification, localization and characterization of the human gamma-synuclein gene. Hum Genet. 1998; 103:106–112.
Article16. Zhao W, Liu H, Liu W, Wu Y, Chen W, Jiang B, et al. Abnormal activation of the synuclein-gamma gene in hepatocellular carcinomas by epigenetic alteration. Int J Oncol. 2006; 28:1081–1088.
Article17. Zhou CQ, Liu S, Xue LY, Wang YH, Zhu HX, Lu N, et al. Down-regulation of gamma-synuclein in human esophageal squamous cell carcinoma. World J Gastroenterol. 2003; 9:1900–1903.
Article18. Liu C, Guo J, Qu L, Bing D, Meng L, Wu J, et al. Applications of novel monoclonal antibodies specific for synuclein-gamma in evaluating its levels in sera and cancer tissues from colorectal cancer patients. Cancer Lett. 2008; 269:148–158.
Article19. Li Z, Sclabas GM, Peng B, Hess KR, Abbruzzese JL, Evans DB, et al. Overexpression of synuclein-gamma in pancreatic adenocarcinoma. Cancer. 2004; 101:58–65.
Article20. Iwaki H, Kageyama S, Isono T, Wakabayashi Y, Okada Y, Yoshimura K, et al. Diagnostic potential in bladder cancer of a panel of tumor markers (calreticulin, gamma-synuclein, and catechol-o-methyltransferase) identified by proteomic analysis. Cancer Sci. 2004; 95:955–961.
Article21. Fung KM, Rorke LB, Giasson B, Lee VM, Trojanowski JQ. Expression of alpha-, beta-, and gamma-synuclein in glial tumors and medulloblastomas. Acta Neuropathol. 2003; 106:167–175.22. Jiang Y, Liu YE, Lu A, Gupta A, Goldberg ID, Liu J, et al. Stimulation of estrogen receptor signaling by gamma synuclein. Cancer Res. 2003; 63:3899–3903.23. Gupta A, Inaba S, Wong OK, Fang G, Liu J. Breast cancer-specific gene 1 interacts with the mitotic checkpoint kinase BubR1. Oncogene. 2003; 22:7593–7599.
Article24. Inaba S, Li C, Shi YE, Song DQ, Jiang JD, Liu J. Synuclein gamma inhibits the mitotic checkpoint function and promotes chromosomal instability of breast cancer cells. Breast Cancer Res Treat. 2005; 94:25–35.
Article25. Zhang W, Ohnishi K, Shigeno K, Fujisawa S, Naito K, Nakamura S, et al. The induction of apoptosis and cell cycle arrest by arsenic trioxide in lymphoid neoplasms. Leukemia. 1998; 12:1383–1391.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dioscin Decreases Breast Cancer Stem-like Cell Proliferation via Cell Cycle Arrest by Modulating p38 Mitogen-activated Protein Kinase and AKT/mTOR Signaling Pathways
- Action and Signaling of Lysophosphatidylethanolamine in MDA-MB-231 Breast Cancer Cells
- Nuclear Localization of Fibroblast Growth Factor Receptor 1 in Breast Cancer Cells Interacting with Cancer Associated Fibroblasts
- Delphinidin inhibits cell proliferation and induces apoptosis in MDA-MB-231 human breast cancer cell lines
- Inhibition of the Interleukin-11-STAT3 Axis Attenuates Hypoxia-Induced Migration and Invasion in MDA-MB-231 Breast Cancer Cells