J Bone Metab.
2013 May;20(1):31-35. 10.11005/jbm.2013.20.1.31.
Effects of a 'Drug Holiday' on Bone Mineral Density and Bone Turnover Marker During Bisphosphonate Therapy
- Affiliations
-
- 1Department of Internal Medicine, Cheil General Hospital & Women's Healthcare Center, Kwandong University College of Medicine, Seoul, Korea. hyunkooyoon@hotmail.com
- KMID: 2286310
- DOI: http://doi.org/10.11005/jbm.2013.20.1.31
Abstract
- BACKGROUND
Recently long-term safety of bisphosphonate raises issues about the duration of therapy. We examined the effects of a drug holiday (DH) on bone mineral density (BMD) and bone turnover markers.
METHODS
In Korean, 125 women of 50 years of age or older with T-score< or =-3.0 of their lumbar or left femoral BMD initiated bisphosphonate from 1999 based on retrospective chart review. 125 patients who had used bisphosphonate> or =5 years started DH in 2006. Lumbar (L1-4), left femoral neck, total BMD, serum parameter (beta-crossLaps [CTx], phosphorus, total calcium, total alkaline phosphatase), and urinary parameter (calcium/creatinine ratio) were measured before, the time of starting, and after DH.
RESULTS
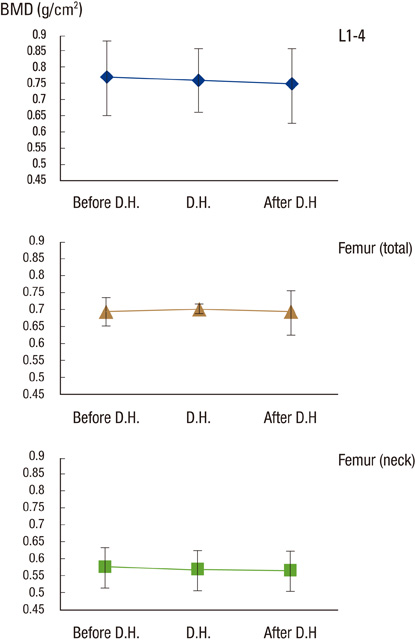
After DH, lumbar, femoral neck and total BMD did not change significantly (0.757+/-0.093-->0.747+/-0.102, P=0.135, 0.567+/-0.079-->0.560+/-0.082, P=0.351, 0.698+/-0.008-->0.691+/-0.090 g/cm2, P=0.115, respectively). Serum CTx and total alkaline phosphatase were increased significantly (0.205+/-0.120-->0.791+/-0.44 ng/mL, P<0.001, 54.52+/-13.40-->60.42+/-15.543 IU/L, P=0.001, respectively). Urinary calcium/creatinine ratio increased significantly (0.132+/-0.076-->0.156+/-0.093, P=0.012).
CONCLUSIONS
A DH could be cautiously considered in patients with long-term use of bisphosphonate if there is a concern about severe suppression of bone turnover with respect to long-term use because insignificant changes of BMD and significant increase of bone turnover markers are shown during the period.
MeSH Terms
Figure
Cited by 1 articles
-
Oral Bisphosphonate and Risk of Esophageal Cancer: A Nationwide Claim Study
Gi Hyeon Seo, Hyung Jin Choi
J Bone Metab. 2015;22(2):77-81. doi: 10.11005/jbm.2015.22.2.77.
Reference
-
1. Gass M, Dawson-Hughes B. Preventing osteoporosis-related fractures: an overview. Am J Med. 2006. 119:S3–S11.
Article2. McCombs JS, Thiebaud P, McLaughlin-Miley C, et al. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004. 48:271–287.
Article3. Caro JJ, Ishak KJ, Huybrechts KF, et al. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004. 15:1003–1008.
Article4. Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006. 81:1013–1022.
Article5. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003. 61:1115–1117.
Article6. Odvina CV, Zerwekh JE, Rao DS, et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005. 90:1294–1301.
Article7. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007. 356:1809–1822.
Article8. Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007. 89:349–353.9. Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009. 360:89–90.
Article10. Ott SM. Long-term safety of bisphosphonates. J Clin Endocrinol Metab. 2005. 90:1897–1899.
Article11. Sebba A. Osteoporosis: how long should we treat? Curr Opin Endocrinol Diabetes Obes. 2008. 15:502–507.
Article12. Watts NB, Chines A, Olszynski WP, et al. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int. 2008. 19:365–372.
Article13. Papapoulos SE, Cremers SC. Prolonged bisphosphonate release after treatment in children. N Engl J Med. 2007. 356:1075–1076.
Article14. Diab DL, Watts NB. Bisphosphonates in the treatment of osteoporosis. Endocrinol Metab Clin North Am. 2012. 41:487–506.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Correlation of Combined Biochemical Markers of Bone Turnover for Bone Mineral Density in Postmenopausal women
- Increase in the Serum Parathyroid Hormone Level During a Bisphosphonate Drug Holiday
- Effect of Soybean Intake on Bone Mineral Density and Bone Turnover Markers in Postmenopausal Women
- The Effects of Low-Dose Bisphosphonate Treatment on Bone Mineral Density and Bone Turnover Markers in Elderly Patients With Osteoporosis
- Effect of Intravenous Administration of Bisphosphonate for Patients Operatively Treated for Osteoporotic Hip Fracture