Korean J Physiol Pharmacol.
2016 May;20(3):315-324. 10.4196/kjpp.2016.20.3.315.
Effects of hydrogen peroxide on voltage-dependent K+ currents in human cardiac fibroblasts through protein kinase pathways
- Affiliations
-
- 1Department of Physiology, College of Medicine, Chung-Ang University, Seoul 06974, Korea. injalim@cau.ac.kr
- 2Biomedical Research Institute, College of Medicine, Chung-Ang University, Seoul 06974, Korea.
- 3Department of Internal Medicine, College of Medicine, Chung-Ang University, Seoul 06974, Korea.
- 4Department of Biochemistry, School of Medicine, Konkuk University, Seoul 05029, Korea.
- KMID: 2285604
- DOI: http://doi.org/10.4196/kjpp.2016.20.3.315
Abstract
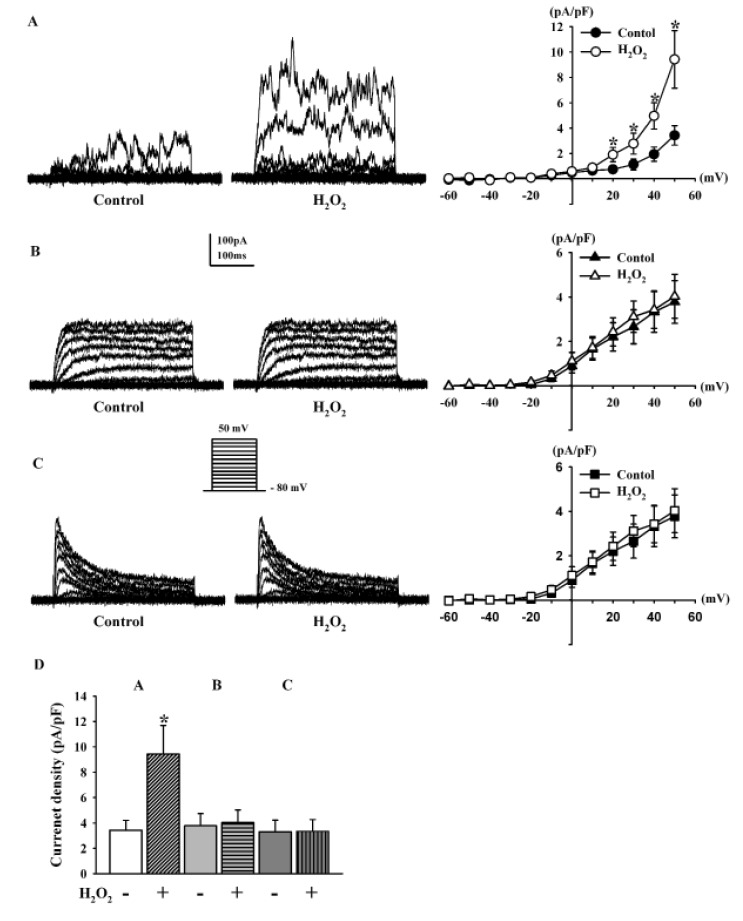
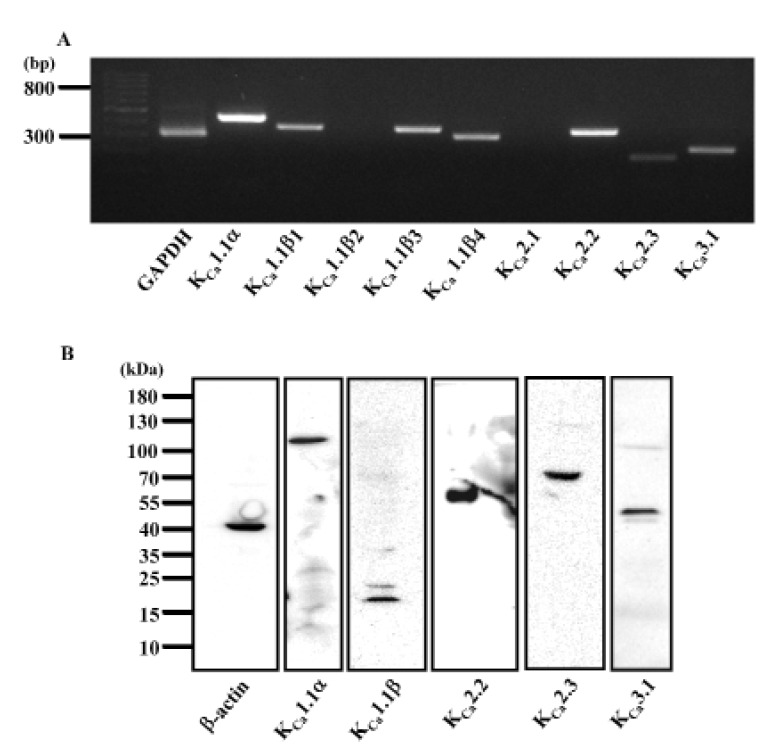
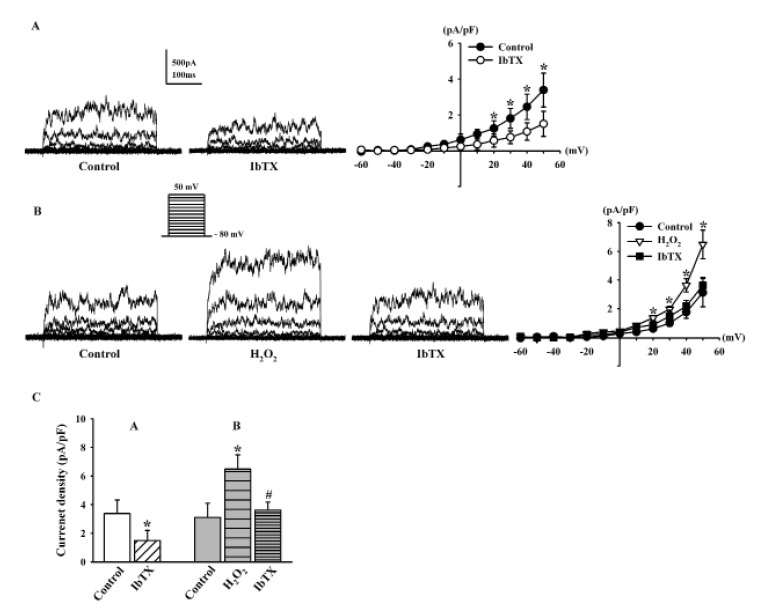
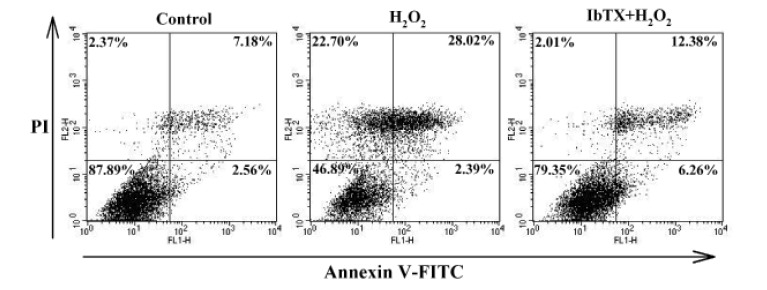
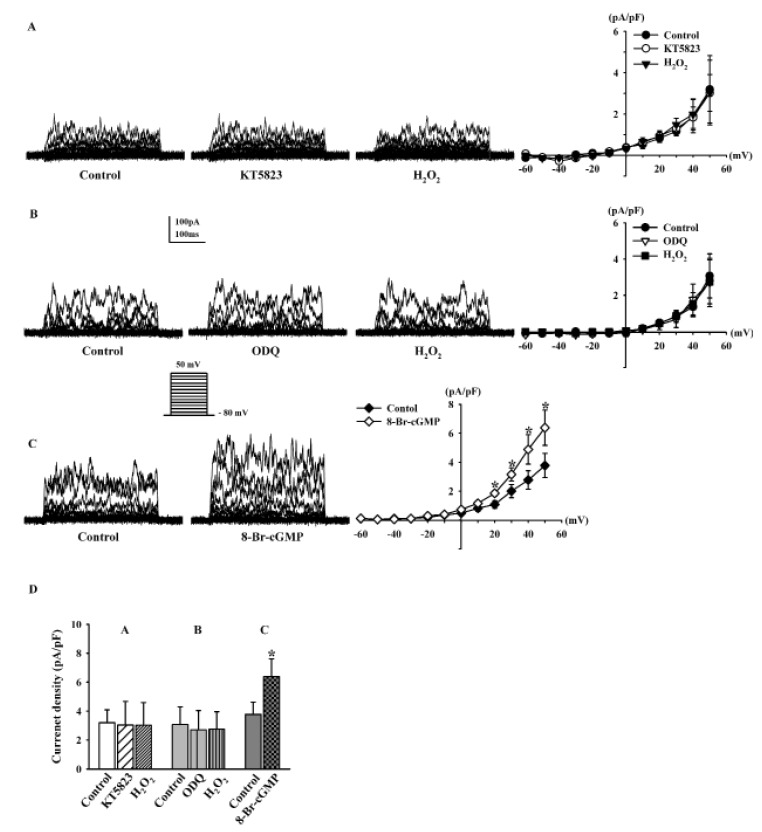
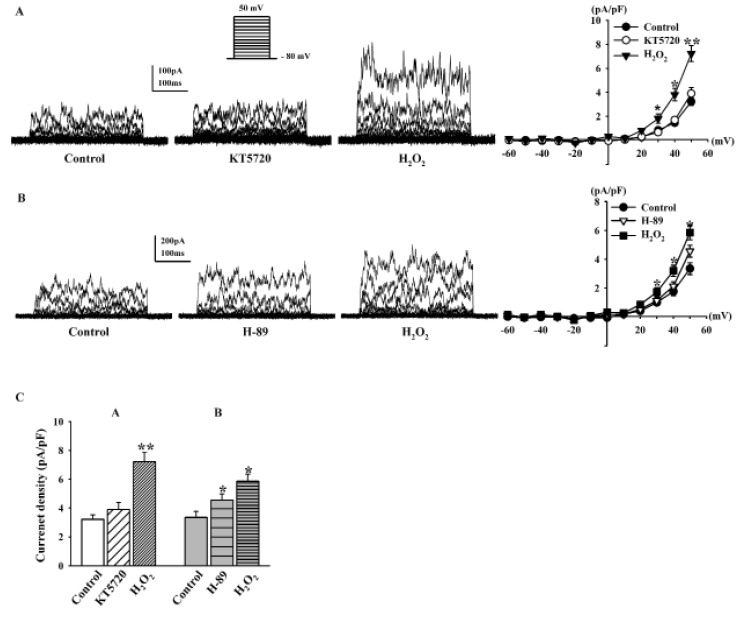
- Human cardiac fibroblasts (HCFs) have various voltage-dependent K+ channels (VDKCs) that can induce apoptosis. Hydrogen peroxide (H2O2) modulates VDKCs and induces oxidative stress, which is the main contributor to cardiac injury and cardiac remodeling. We investigated whether H2O2 could modulate VDKCs in HCFs and induce cell injury through this process. In whole-cell mode patch-clamp recordings, application of H2O2 stimulated Ca2+-activated K+ (K(Ca)) currents but not delayed rectifier K+ or transient outward K+ currents, all of which are VDKCs. H2O2-stimulated K(Ca) currents were blocked by iberiotoxin (IbTX, a large conductance K(Ca) blocker). The H2O2-stimulating effect on large-conductance K(Ca) (BK(Ca)) currents was also blocked by KT5823 (a protein kinase G inhibitor) and 1 H-[1, 2, 4] oxadiazolo-[4, 3-a] quinoxalin-1-one (ODQ, a soluble guanylate cyclase inhibitor). In addition, 8-bromo-cyclic guanosine 3', 5'-monophosphate (8-Br-cGMP) stimulated BK(Ca) currents. In contrast, KT5720 and H-89 (protein kinase A inhibitors) did not block the H2O2-stimulating effect on BK(Ca) currents. Using RT-PCR and western blot analysis, three subtypes of K(Ca) channels were detected in HCFs: BK(Ca) channels, small-conductance K(Ca) (SK(Ca)) channels, and intermediate-conductance K(Ca) (IK(Ca)) channels. In the annexin V/propidium iodide assay, apoptotic changes in HCFs increased in response to H2O2, but IbTX decreased H2O2-induced apoptosis. These data suggest that among the VDKCs of HCFs, H2O2 only enhances BK(Ca) currents through the protein kinase G pathway but not the protein kinase A pathway, and is involved in cell injury through BK(Ca) channels.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
Cyclic AMP-Dependent Protein Kinases
Cyclic GMP-Dependent Protein Kinases
Fibroblasts*
Guanosine
Guanylate Cyclase
Humans*
Hydrogen Peroxide*
Hydrogen*
Oxidative Stress
Phosphotransferases
Potassium Channels, Calcium-Activated
Protein Kinases*
Cyclic AMP-Dependent Protein Kinases
Cyclic GMP-Dependent Protein Kinases
Guanosine
Guanylate Cyclase
Hydrogen
Hydrogen Peroxide
Phosphotransferases
Potassium Channels, Calcium-Activated
Protein Kinases
Figure
Reference
-
1. Dixon IM, Cunnington RH. Mast cells and cardiac fibroblasts: accomplices in elevation of collagen synthesis in modulation of fibroblast phenotype. Hypertension. 2011; 58:142–144. PMID: 21730295.2. Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005; 65:40–51. PMID: 15621032.
Article3. Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010; 225:631–637. PMID: 20635395.
Article4. Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005; 45:657–687. PMID: 15822192.
Article5. Flack EC, Lindsey ML, Squires CE, Kaplan BS, Stroud RE, Clark LL, Escobar PG, Yarbrough WM, Spinale FG. Alterations in cultured myocardial fibroblast function following the development of left ventricular failure. J Mol Cell Cardiol. 2006; 40:474–483. PMID: 16516916.6. Kohl P, Camelliti P. Cardiac myocyte-nonmyocyte electrotonic coupling: implications for ventricular arrhythmogenesis. Heart Rhythm. 2007; 4:233–235. PMID: 17275764.
Article7. Kohl P, Camelliti P, Burton FL, Smith GL. Electrical coupling of fibroblasts and myocytes: relevance for cardiac propagation. J Electrocardiol. 2005; 38(4 Suppl):45–50. PMID: 16226073.
Article8. Li GR, Sun HY, Chen JB, Zhou Y, Tse HF, Lau CP. Characterization of multiple ion channels in cultured human cardiac fibroblasts. PLoS One. 2009; 4:e7307. PMID: 19806193.
Article9. Feng B, Ye WL, Ma LJ, Fang Y, Mei YA, Wei SM. Hydrogen peroxide enhanced Ca2+-activated BK currents and promoted cell injury in human dermal fibroblasts. Life Sci. 2012; 90:424–431. PMID: 22273755.10. Jiao S, Wu MM, Hu CL, Zhang ZH, Mei YA. Melatonin receptor agonist 2-iodomelatonin prevents apoptosis of cerebellar granule neurons via K+ current inhibition. J Pineal Res. 2004; 36:109–116. PMID: 14962062.11. Wu CT, Qi XY, Huang H, Naud P, Dawson K, Yeh YH, Harada M, Kuo CT, Nattel S. Disease and region-related cardiac fibroblast potassium current variations and potential functional significance. Cardiovasc Res. 2014; 102:487–496. PMID: 24596399.
Article12. Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997; 278:114–117. PMID: 9311914.
Article13. Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011; 89:744–753. PMID: 20962103.
Article14. Toro L, Wallner M, Meera P, Tanaka Y. Maxi-KCa, a unique member of the voltage-gated K channel superfamily. News Physiol Sci. 1998; 13:112–117. PMID: 11390773.15. Hoffman JF, Joiner W, Nehrke K, Potapova O, Foye K, Wickrema A. The hSK4 (KCNN4) isoform is the Ca2+-activated K+ channel (Gardos channel) in human red blood cells. Proc Natl Acad Sci U S A. 2003; 100:7366–7371. PMID: 12773623.16. Ouadid-Ahidouch H, Roudbaraki M, Delcourt P, Ahidouch A, Joury N, Prevarskaya N. Functional and molecular identification of intermediate-conductance Ca2+-activated K+ channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol. 2004; 287:C125–C134. PMID: 14985237.17. Sheng JZ, Braun AP. Small- and intermediate-conductance Ca2+-activated K+ channels directly control agonist-evoked nitric oxide synthesis in human vascular endothelial cells. Am J Physiol Cell Physiol. 2007; 293:C458–C467. PMID: 17459950.18. Mathie A, Wooltorton JR, Watkins CS. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. Gen Pharmacol. 1998; 30:13–24. PMID: 9457476.
Article19. Park WS, Firth AL, Han J, Ko EA. Patho-, physiological roles of voltage-dependent K+ channels in pulmonary arterial smooth muscle cells. J Smooth Muscle Res. 2010; 46:89–105. PMID: 20551590.20. Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res. 2009; 81:482–490. PMID: 19050008.
Article21. Angelova P, Muller W. Oxidative modulation of the transient potassium current IA by intracellular arachidonic acid in rat CA1 pyramidal neurons. Eur J Neurosci. 2006; 23:2375–2384. PMID: 16706845.22. Dong DL, Yue P, Yang BF, Wang WH. Hydrogen peroxide stimulates the Ca2+-activated big-conductance K channels (BK) through cGMP signaling pathway in cultured human endothelial cells. Cell Physiol Biochem. 2008; 22:119–126. PMID: 18769038.
Article23. Dong DL, Liu Y, Zhou YH, Song WH, Wang H, Yang BF. Decreases of voltage-dependent K+ currents densities in ventricular myocytes of guinea pigs by chronic oxidant stress. Acta Pharmacol Sin. 2004; 25:751–755. PMID: 15169627.24. Huang SY, Lu YY, Chen YC, Chen WT, Lin YK, Chen SA, Chen YJ. Hydrogen peroxide modulates electrophysiological characteristics of left atrial myocytes. Acta Cardiol Sin. 2014; 30:38–45. PMID: 27122766.25. Wang YJ, Sung RJ, Lin MW, Wu SN. Contribution of BKCa-channel activity in human cardiac fibroblasts to electrical coupling of cardiomyocytes-fibroblasts. J Membr Biol. 2006; 213:175–185. PMID: 17483867.26. Chilton L, Ohya S, Freed D, George E, Drobic V, Shibukawa Y, Maccannell KA, Imaizumi Y, Clark RB, Dixon IM, Giles WR. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am J Physiol Heart Circ Physiol. 2005; 288:H2931–H2939. PMID: 15653752.27. Brzezinska AK, Gebremedhin D, Chilian WM, Kalyanaraman B, Elliott SJ. Peroxynitrite reversibly inhibits Ca2+-activated K+ channels in rat cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000; 278:H1883–H1890. PMID: 10843885.28. Rogers PA, Chilian WM, Bratz IN, Bryan RM Jr, Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive KV channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007; 292:H1404–H1411. PMID: 17071731.29. Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ Res. 2012; 110:471–480. PMID: 22158710.30. Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000; 106:1521–1530. PMID: 11120759.
Article31. Lim I, Yun J, Kim S, Lee C, Seo S, Kim T, Bang H. Nitric oxide stimulates a large-conductance Ca-activated K+ channel in human skin fibroblasts through protein kinase G pathway. Skin Pharmacol Physiol. 2005; 18:279–287. PMID: 16145282.32. Roh S, Choi S, Lim I. Involvement of protein kinase A in nitric oxide stimulating effect on a BKCa channel of human dermal fibroblasts. J Invest Dermatol. 2007; 127:2533–2538. PMID: 17554366.33. Park WS, Son YK, Kim N, Youm JB, Warda M, Ko JH, Ko EA, Kang SH, Kim E, Earm YE, Han J. Direct modulation of Ca2+-activated K+ current by H-89 in rabbit coronary arterial smooth muscle cells. Vascul Pharmacol. 2007; 46:105–113. PMID: 17052962.34. Krick S, Platoshyn O, Sweeney M, McDaniel SS, Zhang S, Rubin LJ, Yuan JX. Nitric oxide induces apoptosis by activating K+ channels in pulmonary vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2002; 282:H184–H193. PMID: 11748062.35. Ma YG, Dong L, Ye XL, Deng CL, Cheng JH, Liu WC, Ma J, Chang YM, Xie MJ. Activation of cloned BKCa channels in nitric oxide-induced apoptosis of HEK293 cells. Apoptosis. 2010; 15:426–438. PMID: 20012488.36. Yun J, Park H, Ko JH, Lee W, Kim K, Kim T, Shin J, Kim K, Kim K, Song JH, Noh YH, Bang H, Lim I. Expression of Ca2+ -activated K+ channels in human dermal fibroblasts and their roles in apoptosis. Skin Pharmacol Physiol. 2010; 23:91–104. PMID: 20016251.37. Choi S, Lee W, Yun J, Seo J, Lim I. Expression of Ca-activated K channels and their role in proliferation of rat cardiac fibroblasts. Korean J Physiol Pharmacol. 2008; 12:51–58. PMID: 20157394.38. Wang LP, Wang Y, Zhao LM, Li GR, Deng XL. Angiotensin II upregulates KCa3.1 channels and stimulates cell proliferation in rat cardiac fibroblasts. Biochem Pharmacol. 2013; 85:1486–1494. PMID: 23500546.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Pacemaker Activity of Interstitial Cells of Cajal by Hydrogen Peroxide via Activating ATP-sensitive K(+) Channels
- Effects of Tamoxifen on the Voltage-dependent Ionic Currents in Mouse Colonic Smooth Muscle Cells
- Carbon monoxide activation of delayed rectifier potassium currents of human cardiac fibroblasts through diverse pathways
- The Effects of Hydrogen Peroxide on the Migration and Proliferation of the Human Keratinocytes during Wound Healing
- Effects of Propofol in Voltage-dependent Potassium Channels in Human Neurl Stem Cells