Korean J Physiol Pharmacol.
2015 Sep;19(5):461-465. 10.4196/kjpp.2015.19.5.461.
Toll-like Receptor 2 is Dispensable for an Immediate-early Microglial Reaction to Two-photon Laser-induced Cortical Injury In vivo
- Affiliations
-
- 1Department of Physiology, College of Korean Medicine, Kyung Hee University, Seoul 130-701, Korea. skkim77@khu.ac.kr
- 2Department of Oral Physiology and Neuroscience, School of Dentistry, Seoul National University, Seoul 110-749, Korea. sjlee87@snu.ac.kr
- 3Department of Physiology, School of Medicine, Seoul National University, Seoul 110-799, Korea. sangjkim@snu.ac.kr
- KMID: 2285591
- DOI: http://doi.org/10.4196/kjpp.2015.19.5.461
Abstract
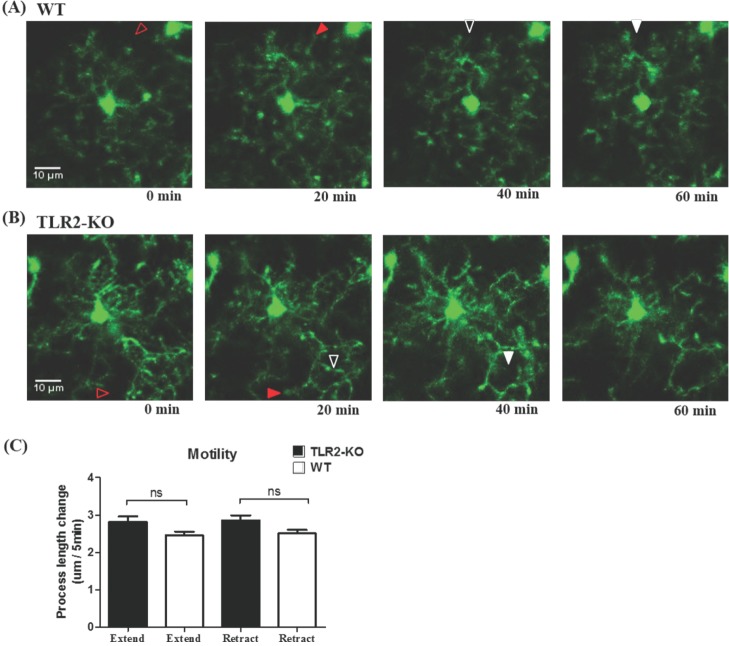
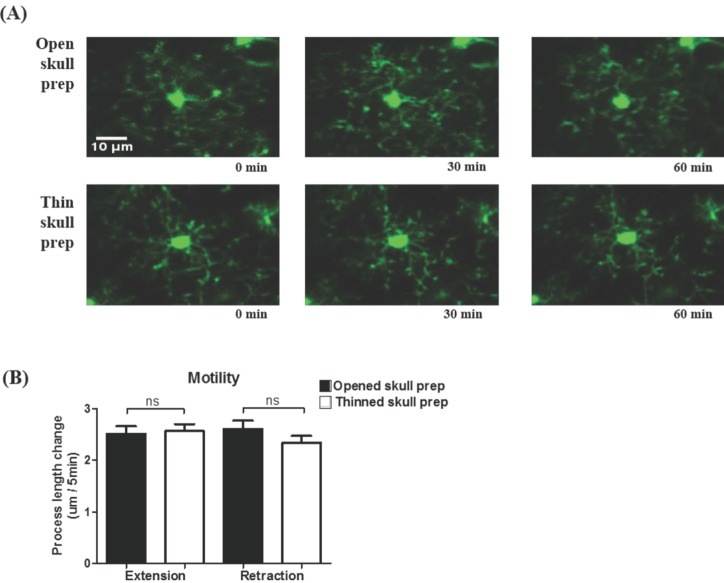
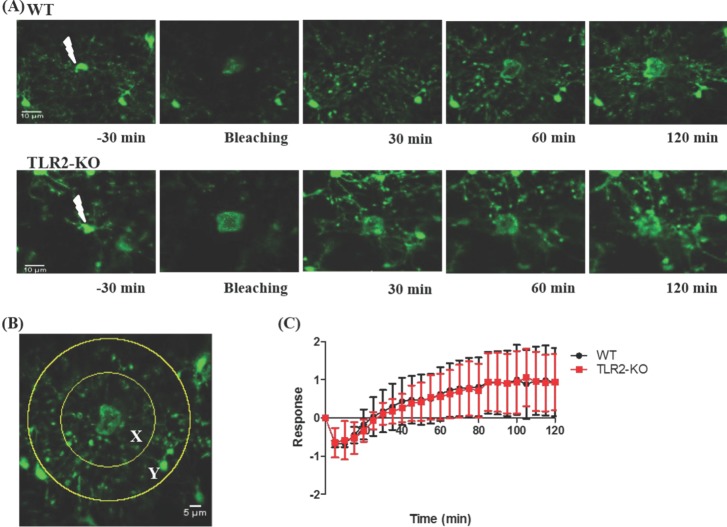
- Microglia, the resident macrophages in the central nervous system, can rapidly respond to pathological insults. Toll-like receptor 2 (TLR2) is a pattern recognition receptor that plays a fundamental role in pathogen recognition and activation of innate immunity. Although many previous studies have suggested that TLR2 contributes to microglial activation and subsequent pathogenesis following brain tissue injury, it is still unclear whether TLR2 has a role in microglia dynamics in the resting state or in immediate-early reaction to the injury in vivo. By using in vivo two-photon microscopy imaging and Cx3cr1(GFP/+) mouse line, we first monitored the motility of microglial processes (i.e. the rate of extension and retraction) in the somatosensory cortex of living TLR2-KO and WT mice; Microglial processes in TLR2-KO mice show the similar motility to that of WT mice. We further found that microglia rapidly extend their processes to the site of local tissue injury induced by a two-photon laser ablation and that such microglial response to the brain injury was similar between WT and TLR2-KO mice. These results indicate that there are no differences in the behavior of microglial processes between TLR2-KO mice and WT mice when microglia is in the resting state or encounters local injury. Thus, TLR2 might not be essential for immediate-early microglial response to brain tissue injury in vivo.
MeSH Terms
Figure
Reference
-
1. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007; 10:1387–1394. PMID: 17965659.
Article3. Petersen MA, Dailey ME. Diverse microglial motility behaviors during clearance of dead cells in hippocampal slices. Glia. 2004; 46:195–206. PMID: 15042586.
Article4. Stence N, Waite M, Dailey ME. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. 2001; 33:256–266. PMID: 11241743.
Article5. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005; 308:1314–1318. PMID: 15831717.
Article6. Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005; 8:752–758. PMID: 15895084.7. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–384. PMID: 20404851.
Article8. Lehnardt S, Henneke P, Lien E, Kasper DL, Volpe JJ, Bechmann I, Nitsch R, Weber JR, Golenbock DT, Vartanian T. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol. 2006; 177:583–592. PMID: 16785556.
Article9. Hayward JH, Lee SJ. A Decade of research on TLR2 discovering its pivotal role in glial activation and neuroinflammation in neurodegenerative diseases. Exp Neurobiol. 2014; 23:138–147. PMID: 24963278.
Article10. Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007; 282:14975–14983. PMID: 17355971.
Article11. Hong J, Cho IH, Kwak KI, Suh EC, Seo J, Min HJ, Choi SY, Kim CH, Park SH, Jo EK, Lee S, Lee KE, Lee SJ. Microglial Toll-like receptor 2 contributes to kainic acid-induced glial activation and hippocampal neuronal cell death. J Biol Chem. 2010; 285:39447–39457. PMID: 20923777.
Article12. Park C, Cho IH, Kim D, Jo EK, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. Toll-like receptor 2 contributes to glial cell activation and heme oxygenase-1 expression in traumatic brain injury. Neurosci Lett. 2008; 431:123–128. PMID: 18164130.
Article13. Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009; 29:3974–3980. PMID: 19339593.14. Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hübener M, Keck T, Knott G, Lee WC, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc. 2009; 4:1128–1144. PMID: 19617885.
Article15. Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010; 5:201–208. PMID: 20134419.
Article16. Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci. 2007; 10:549–551. PMID: 17417634.17. Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992; 149:2736–2741. PMID: 1383325.18. Giulian D, Vaca K, Corpuz M. Brain glia release factors with opposing actions upon neuronal survival. J Neurosci. 1993; 13:29–37. PMID: 8423475.
Article19. Hur J, Lee P, Kim MJ, Cho YW. Regulatory effect of 25-hydroxyvitamin D3 on nitric oxide production in activated microglia. Korean J Physiol Pharmacol. 2014; 18:397–402. PMID: 25352759.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prothrombin Kringle-2: A Potential Inflammatory Pathogen in the Parkinsonian Dopaminergic System
- Targeting Microglial and Neuronal Toll-like Receptor 2 in Synucleinopathies
- Role of microglial activation on neuronal excitability in rat substantia gelatinosa
- Nucleic Acid Recognition and Signaling by Toll-like Receptor 9: Compartment-dependent Regulation
- Toll-like receptor 4 antagonist and obesity associated kidney disease: Where should we go from here?