Korean J Physiol Pharmacol.
2014 Dec;18(6):461-467. 10.4196/kjpp.2014.18.6.461.
Streptozotocin Diabetes Attenuates the Effects of Nondepolarizing Neuromuscular Relaxants on Rat Muscles
- Affiliations
-
- 1Department of Anesthesiology, The Affiliated First People's Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200080, China. bravespirit.li@gmail.com
- KMID: 2285547
- DOI: http://doi.org/10.4196/kjpp.2014.18.6.461
Abstract
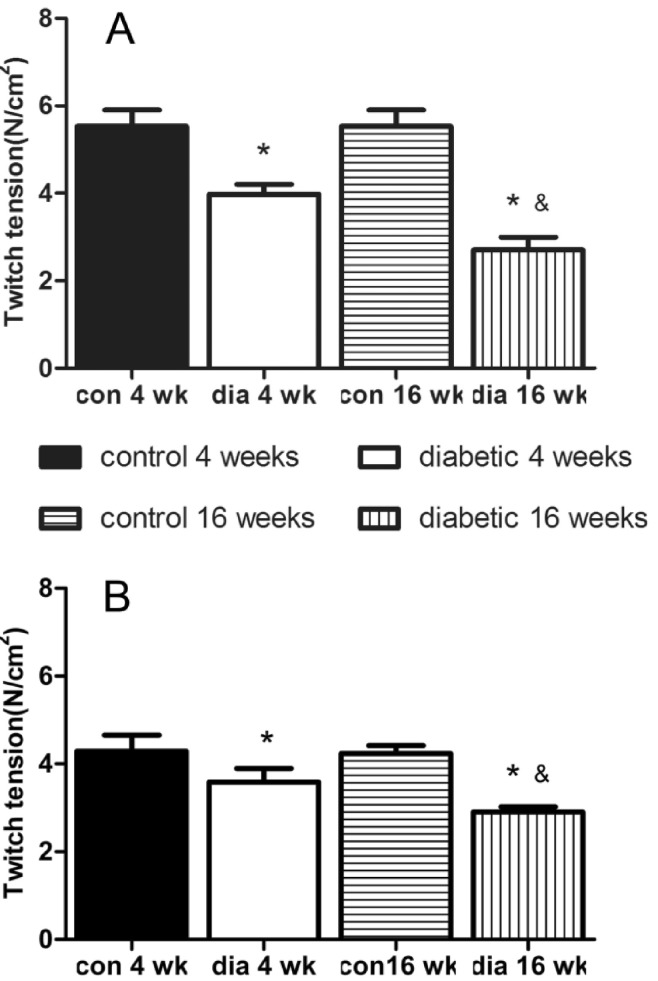
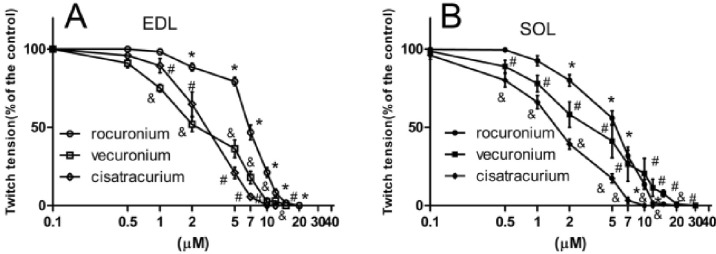
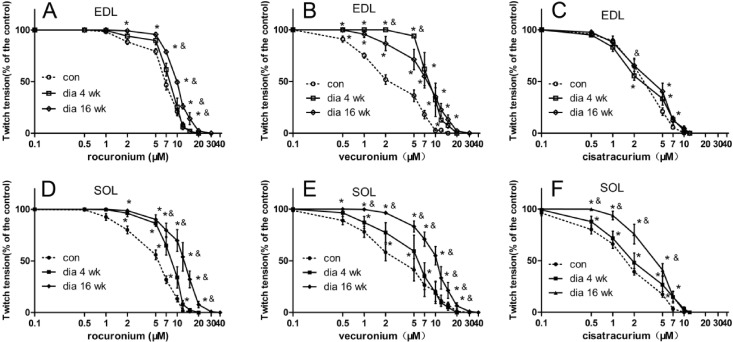
- The hypothesis of this study was that diabetes-induced desensitization of rat soleus (SOL) and extensor digitorum longus (EDL) to non-depolarizing muscle relaxants (NDMRs) depends on the stage of diabetes and on the kind of NDMRs. We tested the different magnitude of resistance to vecuronium, cisatracurium, and rocuronium at different stages of streptozotocin (STZ)-induced diabetes by the EDL sciatic nerve-muscle preparations, and the SOL sciatic nerve-muscle preparations from rats after 4 and 16 weeks of STZ treatment. The concentration-twitch tension curves were significantly shifted from those of the control group to the right in the diabetic groups. Concentration giving 50% of maximal inhibition (IC50) was larger in the diabetic groups for all the NDMRs. For rocuronium and cisatracurium in both SOL and EDL, IC50 was significantly larger in diabetic 16 weeks group than those in the diabetic 4 weeks group. For SOL/EDL, the IC50 ratios were significantly largest in the diabetic 16 weeks group, second largest in the diabetic 4 weeks group, and smallest for the control group. Diabetes-induced desensitization to NDMRs depended on the stage of diabetes and on the different kind of muscles observed while was independent on different kind of NDMRs. The resistance to NDMRs was stronger in the later stage of diabetes (16 versus 4 weeks after STZ treatment). Additionally, when monitoring in SOL, diabetes attenuated the actions of neuromuscular blockade more intensely than that in EDL. Nonetheless, the hyposensitivity to NDMRs in diabetes was not relevant for the kind of NDMRs.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The change of signaling pathway on the electrical stimulated contraction in streptozotocin-induced bladder dysfunction of rats
Jong Soo Han, Young Sil Min, Gil Hyung Kim, Sang-hyun Chae, Yoonjin Nam, Jaehwi Lee, Seok-Yong Lee, Uy Dong Sohn
Korean J Physiol Pharmacol. 2018;22(5):577-584. doi: 10.4196/kjpp.2018.22.5.577.
Reference
-
1. Clements RS Jr. Diabetic neuropathy--new concepts of its etiology. Diabetes. 1979; 28:604–611. PMID: 376381.
Article2. Diabetic peripheral neuropathies. Physiopathology and clinical guidelines. Diabetes Res Clin Pract. 1986; 2:183–256. PMID: 3533482.3. Harati Y. Diabetic peripheral neuropathies. Ann Intern Med. 1987; 107:546–559. PMID: 3115161.
Article4. Fahim MA, Hasan MY, Alshuaib WB. Early morphological remodeling of neuromuscular junction in a murine model of diabetes. J Appl Physiol (1985). 2000; 89:2235–2240. PMID: 11090573.
Article5. Marques MJ, Santo Neto H. Acetylcholine receptors and nerve terminal distribution at the neuromuscular junction of non-obese diabetic mice. Anat Rec. 2002; 267:112–119. PMID: 11997879.
Article6. Schofield GG, Furman BL, Marshall IG. The effect of acute alloxan diabetes on the sensitivity of the rat skeletal neuromuscular junction to drugs. Acta Diabetol Lat. 1978; 15:287–293. PMID: 219652.
Article7. Minker E, Kac P, Blazsó G, Koltai M. A study of the origin of altered pharmacological reactivity of synaptic structures caused by diabetes and pretreatment with contrainsular agents. Acta Physiol Hung. 1984; 63:175–183. PMID: 6146239.8. Kimura M, Fujihara M, Nojima H, Kimura I. Hypersensitivity of acetylcholine receptor in diabetic skeletal muscle to neuromuscular blockers: the effect of myotubes cultured with spinal cord or its extract. J Pharmacobiodyn. 1986; 9:29–38. PMID: 2423670.9. Kimura M, Kimura I, Nojima H, Muroi M. Diabetes mellitus-induced hypersensitivity of mouse skeletal muscles to acetylcholine and succinylcholine. Jpn J Pharmacol. 1986; 40:251–256. PMID: 3702147.
Article10. Peter JB, Barnard RJ, Edgerton VR, Gillespie CA, Stempel KE. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972; 11:2627–2633. PMID: 4261555.
Article11. Cotter M, Cameron NE, Lean DR, Robertson S. Effects of long-term streptozotocin diabetes on the contractile and histochemical properties of rat muscles. Q J Exp Physiol. 1989; 74:65–74. PMID: 2524084.
Article12. Brotto M, Brotto L, Jin JP, Nosek TM, Romani A. Temporal adaptive changes in contractility and fatigability of diaphragm muscles from streptozotocin-diabetic rats. J Biomed Biotechnol. 2010; 2010:931903. PMID: 20467472.
Article13. Cotter M, Cameron NE, Lean DR, Robertson S. Effects of long-term streptozotocin diabetes on the contractile and histochemical properties of rat muscles. Q J Exp Physiol. 1989; 74:65–74. PMID: 2524084.
Article14. Secher NH, Rube N, Secher O. Effect of tubocurarine on human soleus and gastrocnemius muscles. Acta Anaesthesiol Scand. 1982; 26:231–234. PMID: 7113631.
Article15. Kitajima T, Ishii K, Kobayashi T, Ogata H. Differential effects of vecuronium on the thumb and great toe as measured by accelography and electromyography. Anaesthesia. 1995; 50:76–78. PMID: 7702151.
Article16. Saitoh Y, Tanaka H, Toyooka H, Amaha K. Recovery of post-tetanic and train-of-four responses at the first dorsal interosseous and adductor pollicis muscles in patients receiving vecuronium. Can J Anaesth. 1996; 43:362–367. PMID: 8697551.
Article17. Saitoh Y, Koitabashi Y, Makita K, Tanaka H, Amaha K. Train-of-four and double burst stimulation fade at the great toe and thumb. Can J Anaesth. 1997; 44:390–395. PMID: 9104521.
Article18. Taquahashi Y, Yonezawa K, Nishimura M. Influence of motor activities on the release of transmitter quanta from motor nerve terminals in mice. J Vet Med Sci. 1999; 61:513–516. PMID: 10379943.
Article19. Sieck DC, Zhan WZ, Fang YH, Ermilov LG, Sieck GC, Mantilla CB. Structure-activity relationships in rodent diaphragm muscle fibers vs. neuromuscular junctions. Respir Physiol Neurobiol. 2012; 180:88–96. PMID: 22063925.
Article20. Wood SJ, Slater CR. The contribution of postsynaptic folds to the safety factor for neuromuscular transmission in rat fastand slow-twitch muscles. J Physiol. 1997; 500:165–176. PMID: 9097941.21. McGuire M, MacDermott M. The influence of streptozotocin diabetes and metformin on erythrocyte volume and on the membrane potential and the contractile characteristics of the extensor digitorum longus and soleus muscles in rats. Exp Physiol. 1999; 84:1051–1058. PMID: 10564702.
Article22. Oishi PE, Cholsiripunlert S, Gong W, Baker AJ, Bernstein HS. Myo-mechanical analysis of isolated skeletal muscle. J Vis Exp. 2011; (48):
Article23. Kimura I, Okazaki M, Kimura M. Streptozocin-diabetes modifies acetylcholine release from mouse phrenic nerve terminal and presynaptic sensitivity to succinylcholine. Jpn J Pharmacol. 1993; 62:35–41. PMID: 8341027.
Article24. Medina Sánchez M, Rodríguez Sánchez C, Vega Alvarez JA, Menéndez Peláez A. Ultrastructural study of neuromuscular junction in rectus femoris muscle of streptozotocin-diabetic rats. Histol Histopathol. 1992; 7:607–610. PMID: 1457982.25. Kimura M, Kimura I, Fujihara M, Hoshino N. Diabetic state-induced modifications of succinylcholine binding mode in the microsomal fractions of mouse skeletal muscles. Life Sci. 1988; 42:1029–1036. PMID: 3343894.
Article26. Saitoh Y, Hattori H, Sanbe N, Nakajima H, Akatu M, Murakawa M. Reversal of vecuronium with neostigmine in patients with diabetes mellitus. Anaesthesia. 2004; 59:750–754. PMID: 15270964.27. Alper I, Ulukaya S, Makay O, Balcioglu T. The pharmacodynamic effects of rocuronium during general anesthesia in patients with type 2 diabetes mellitus. Minerva Anestesiol. 2010; 76:115–119. PMID: 20150852.28. Narimatsu E, Niiya T, Kawamata M, Namiki A. Sepsis stage dependently and differentially attenuates the effects of nondepolarizing neuromuscular blockers on the rat diaphragm in vitro. Anesth Analg. 2005; 100:823–829. PMID: 15728074.
Article29. Lee BH, Shin TJ, Hwang SH, Choi SH, Kang J, Kim HJ, Park CW, Lee SH, Nah SY. Inhibitory effects of quercetin on muscle-type of nicotinic acetylcholine receptor-mediated ion currents expressed in Xenopus Oocytes. Korean J Physiol Pharmacol. 2011; 15:195–201. PMID: 21994477.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Succinylcholine on the Neuromuscular Block Induced with Mivacurium in the abbits
- Clinical Evaluation of Atracurium for Endotracheal Intubation
- Effects of Ondansetron on the Neuromuscular Block of Vecuronium, Rocuronium or Atracurium in a Rat Phrenic Nerve-Hemidiaphragm Preparation
- The Train-of-Four Ratio Profile During Onset and Offset Following Administration of Neuromuscular Blocking Agents
- Postoperative residual neuromuscular blockade