Korean J Physiol Pharmacol.
2014 Apr;18(2):109-120. 10.4196/kjpp.2014.18.2.109.
Multiple Effects of a Novel Epothilone Analog on Cellular Processes and Signaling Pathways Regulated by Rac1 GTPase in the Human Breast Cancer Cells
- Affiliations
-
- 1Research Center for Molecular Medicine, Faculty of Chemical, Environmental and Biological Science and Technology, Dalian University of Technology, Dalian 116023, China. rqiu2001@yahoo.com, tangli63b@yahoo.com
- KMID: 2285499
- DOI: http://doi.org/10.4196/kjpp.2014.18.2.109
Abstract
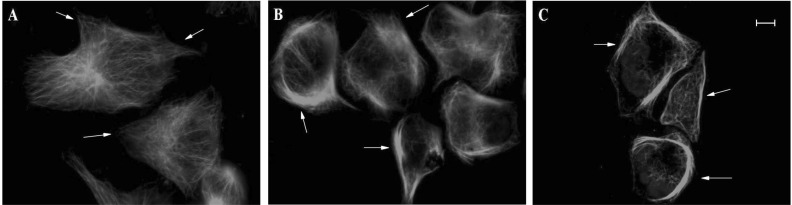
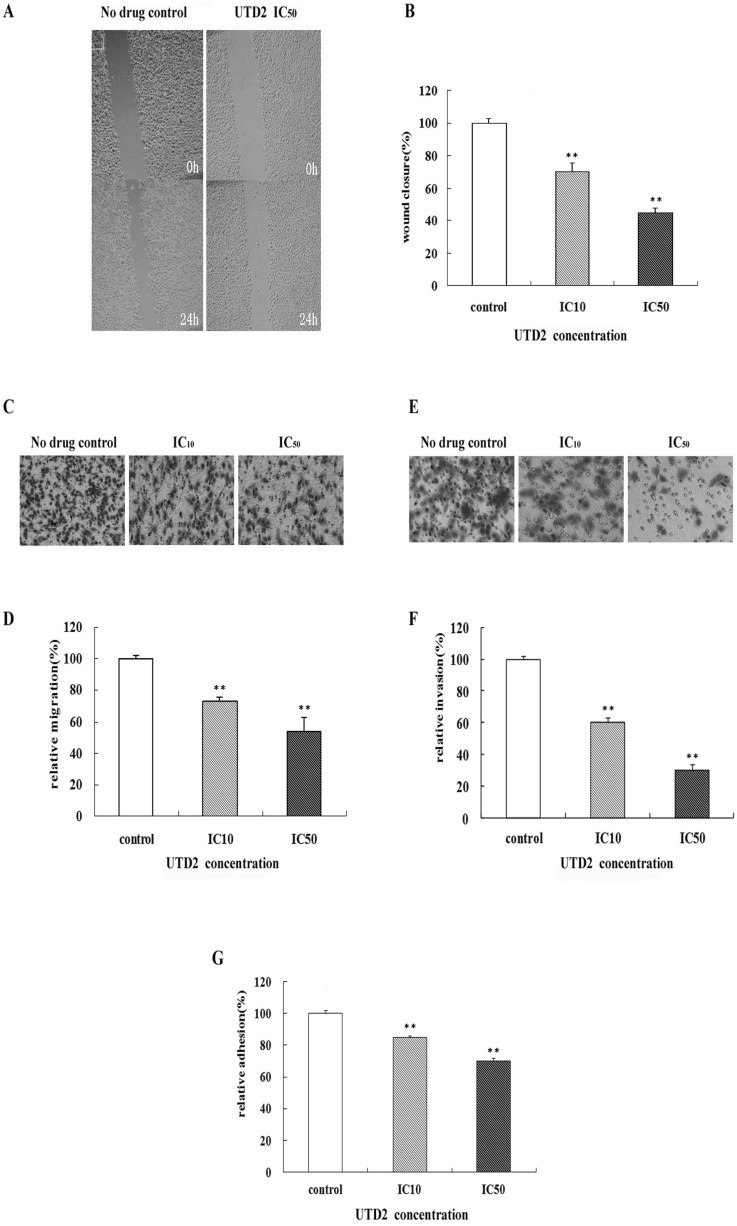
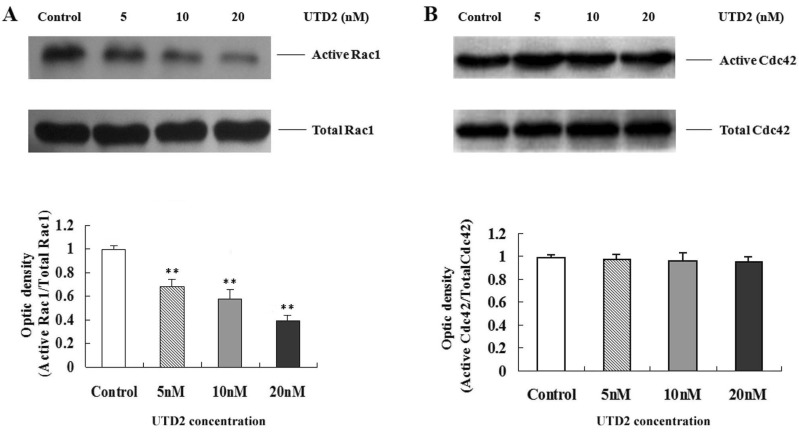
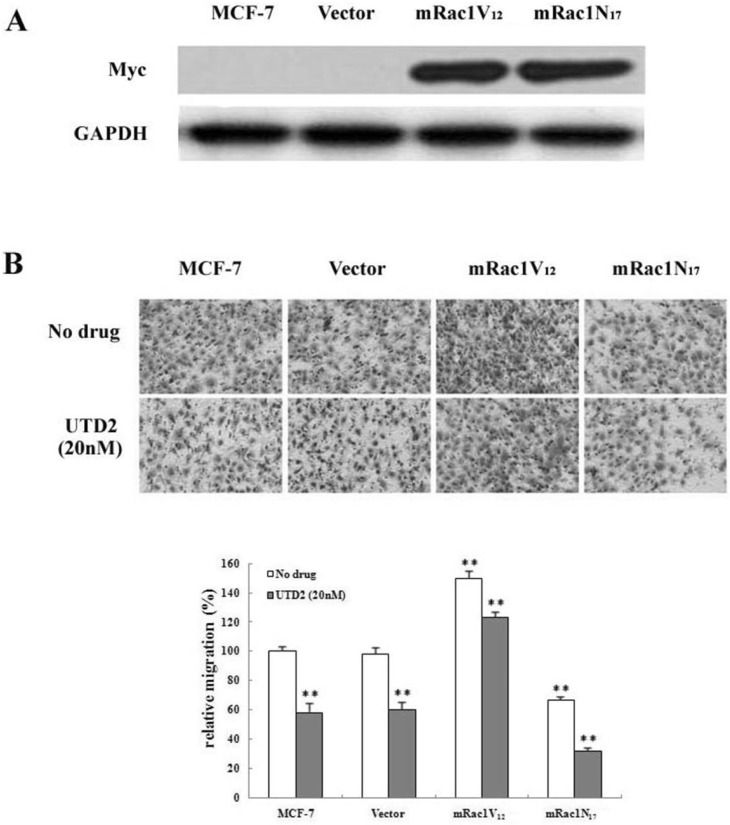
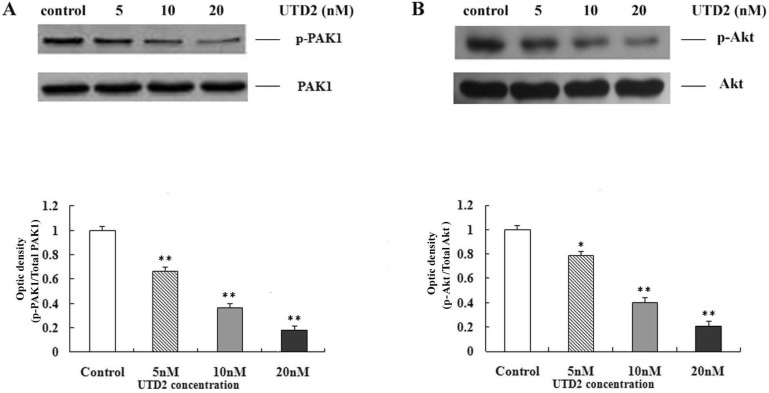
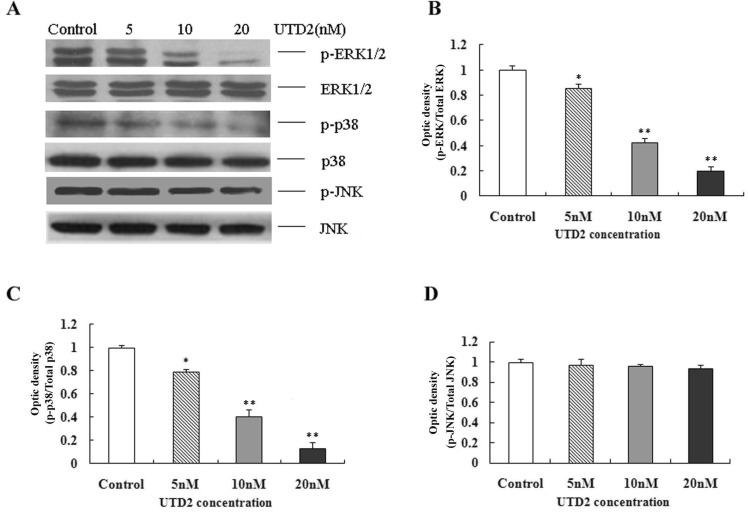
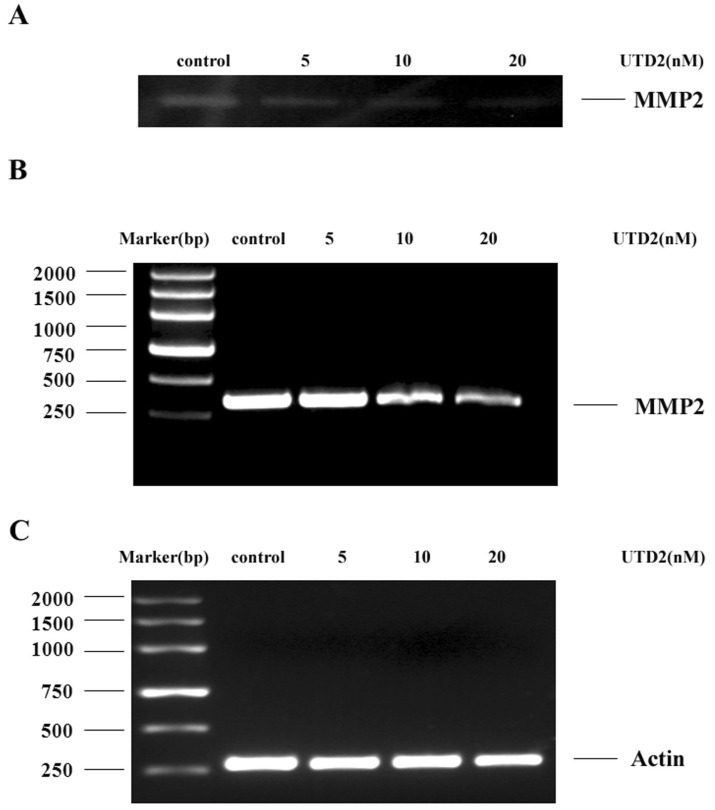
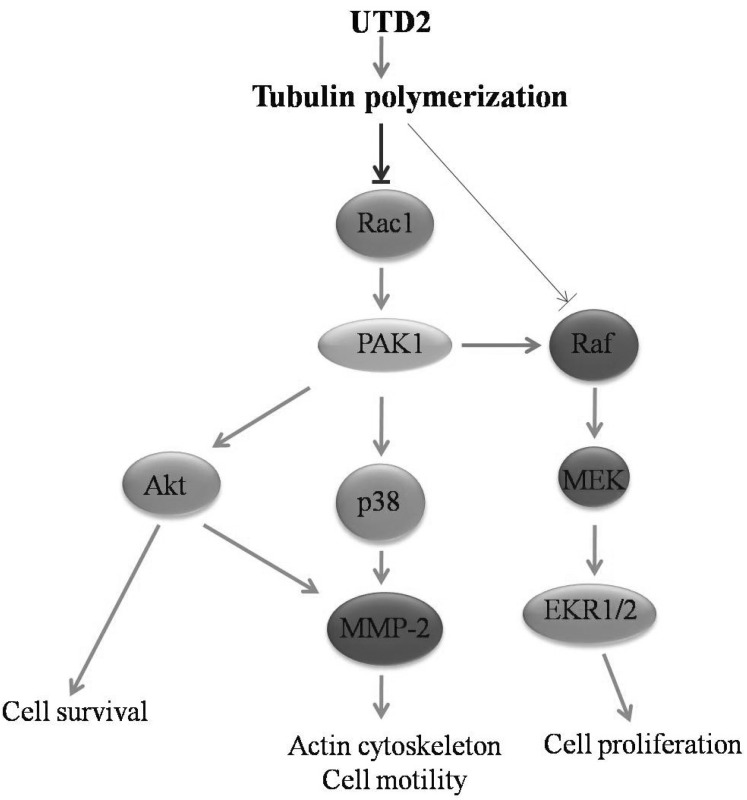
- The epothilones are a class of microtubule inhibitors that exhibit a strong antitumor activity. UTD2 is a novel epothilone analog generated by genetic manipulation of the polyketide biosynthetic gene cluster. This study investigated the effects of UTD2 on the actin cytoskeleton and its critical regulators, and the signaling pathways which are essential for cell motility, growth and survival in MCF-7 breast cancer cells. Results showed that UTD2 inhibited the cellular functions of actin cytoskeleton, such as wound-closure, migration and invasion, as well as adhesion. Our study further demonstrated that UTD2 suppressed Rac1 GTPase activation and reduced the activity of PAK1, which is a downstream effector of Rac1, while the activity of Cdc42 was not affected. Additionally, the phosphorylation of p38 and ERK were significantly inhibited, but the phosphorylation of JNK remained the same after UTD2 treatment. Moreover, UTD2 inhibited the activity and mRNA expression of MMP-2, which plays a key role in cell motility. UTD2 also reduced the phosphorylation of Akt, which is an important signaling kinase regulating the cell survival through Rac1. Furthermore, UTD2 interrupted the synergy between Rac1 and Raf in focus formation assays. Taken together, these results indicated that UTD2 exerted multiple effects on the actin cytoskeleton and signaling pathways associated with Rac1. This study provided novel insights into the molecular mechanism of the antineoplastic and antimetastatic activities of epothilones. Our findings also suggest that the signaling pathways regulated by Rac1 may be evaluated as biomarkers for the response to therapy in clinical trials of epothilones.
Keyword
MeSH Terms
Figure
Reference
-
1. Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995; 55:2325–2333. PMID: 7757983.2. Fumoleau P, Coudert B, Isambert N, Ferrant E. Novel tubulin-targeting agents: anticancer activity and pharmacologic profile of epothilones and related analogues. Ann Oncol. 2007; 18(Suppl 5):v9–v15. PMID: 17656562.
Article3. Buzdar AU. Clinical experience with epothilones in patients with breast cancer. Clin Breast Cancer. 2008; 8(Suppl 2):S71–S78. PMID: 18637402.
Article4. Denduluri N, Swain S. Ixabepilone: clinical role in metastatic breast cancer. Clin Breast Cancer. 2011; 11:139–145. PMID: 21665133.
Article5. Tang Y, Olufemi L, Wang MT, Nie D. Role of Rho GTPases in breast cancer. Front Biosci. 2008; 13:759–776. PMID: 17981586.
Article6. Niu M, Sun Y, Liu B, Tang L, Qiu R. Differential effects of tautomycetin and its derivatives on protein phosphatase inhibition, immunosuppressive function and antitumor activity. Korean J Physiol Pharmacol. 2012; 16:145–151. PMID: 22563261.
Article7. Faull RJ, Kovach NL, Harlan JM, Ginsberg MH. Affinity modulation of integrin alpha 5 beta 1: regulation of the functional response by soluble fibronectin. J Cell Biol. 1993; 121:155–162. PMID: 8458867.
Article8. Saad S, Gottlieb DJ, Bradstock KF, Overall CM, Bendall LJ. Cancer cell-associated fibronectin induces release of matrix metalloproteinase-2 from normal fibroblasts. Cancer Res. 2002; 62:283–289. PMID: 11782389.9. Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995; 374:457–459. PMID: 7700355.
Article10. Ahn JH, Kim MH, Kwon HJ, Choi SY, Kwon HY. Protective effects of oleic acid against palmitic acid-induced apoptosis in pancreatic AR42J cells and its mechanisms. Korean J Physiol Pharmacol. 2013; 17:43–50. PMID: 23440052.
Article11. Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic. 2004; 5:470–477. PMID: 15180824.
Article12. Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002; 420:629–635. PMID: 12478284.
Article14. Menges CW, Sementino E, Talarchek J, Xu J, Chernoff J, Peterson JR, Testa JR. Group I p21-activated kinases (PAKs) promote tumor cell proliferation and survival through the AKT1 and Raf-MAPK pathways. Mol Cancer Res. 2012; 10:1178–1188. PMID: 22798428.
Article15. Xue G, Hemmings BA. PKB/Akt-dependent regulation of cell motility. J Natl Cancer Inst. 2013; 105:393–404. PMID: 23355761.
Article16. Thomas E, Tabernero J, Fornier M, Conteé P, Fumoleau P, Lluch A, Vahdat LT, Bunnell CA, Burris HA, Viens P, Baselga J, Rivera E, Guarneri V, Poulart V, Klimovsky J, Lebwohl D, Martin M. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol. 2007; 25:3399–3406. PMID: 17606975.
Article17. Wittmann T, Waterman-Storer CM. Cell motility: can Rho GTPases and microtubules point the way? J Cell Sci. 2001; 114:3795–3803. PMID: 11719546.
Article18. Hu YL, Li S, Miao H, Tsou TC, del Pozo MA, Chien S. Roles of microtubule dynamics and small GTPase Rac in endothelial cell migration and lamellipodium formation under flow. J Vasc Res. 2002; 39:465–476. PMID: 12566972.
Article19. Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009; 28:51–63. PMID: 19165420.
Article20. Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, Chernoff J, Hung MC, Kumar R. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem. 2000; 275:36238–36244. PMID: 10945974.
Article21. Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem Cell Biol. 2005; 83:1–14. PMID: 15746962.
Article22. Lee KS, Shin JS, Nam KS. Starfish polysaccharides down-regulate metastatic activity through the MAPK signaling pathway in MCF-7 human breast cancer cells. Mol Biol Rep. 2013; 40:5959–5966. PMID: 24065532.
Article23. Lai Y, Liu XH, Zeng Y, Zhang Y, Shen Y, Liu Y. Interleukin-8 induces the endothelial cell migration through the Rac 1/RhoA-p38MAPK pathway. Eur Rev Med Pharmacol Sci. 2012; 16:630–638. PMID: 22774404.24. Guruvayoorappan C, Kuttan G. Amentoflavone inhibits experimental tumor metastasis through a regulatory mechanism involving MMP-2, MMP-9, prolyl hydroxylase, lysyl oxidase, VEGF, ERK-1, ERK-2, STAT-1, NM23 and cytokines in lung tissues of C57BL/6 mice. Immunopharmacol Immunotoxicol. 2008; 30:711–727. PMID: 18686102.
Article25. Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS, Wu SH, Chung JG. Benzyl isothiocyanate (BITC) inhibits migration and invasion of human colon cancer HT29 cells by inhibiting matrix metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC and MAPK signaling pathway. J Agric Food Chem. 2010; 58:2935–2942. PMID: 20136087.
Article26. Ren Y, Wu Q, Liu Y, Xu X, Quan C. Gene silencing of claudin 6 enhances cell proliferation and migration accompanied with increased MMP 2 activity via p38 MAPK signaling pathway in human breast epithelium cell line HBL 100. Mol Med Rep. 2013; 8:1505–1510. PMID: 24026616.27. Osada M, Tolkacheva T, Li W, Chan TO, Tsichlis PN, Saez R, Kimmelman AC, Chan AM. Differential roles of Akt, Rac, and Ral in R-Ras-mediated cellular transformation, adhesion, and survival. Mol Cell Biol. 1999; 19:6333–6344. PMID: 10454580.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rac1 inhibition protects the kidney against kidney ischemia/ reperfusion through the inhibition of macrophage migration
- Signal Transduction Pathways in Colorectal Cancer Carcinogenesis and Metastasis
- Insights into Hypoxia: Non-invasive Assessment through Imaging Modalities and Its Application in Breast Cancer
- Dioscin Decreases Breast Cancer Stem-like Cell Proliferation via Cell Cycle Arrest by Modulating p38 Mitogen-activated Protein Kinase and AKT/mTOR Signaling Pathways
- New Findings on Breast Cancer Stem Cells: A Review