Korean J Physiol Pharmacol.
2013 Dec;17(6):505-510. 10.4196/kjpp.2013.17.6.505.
Electroacupuncture Analgesia Is Improved by Adenoviral Gene Transfer of Dopamine Beta-hydroxylase into the Hypothalamus of Rats
- Affiliations
-
- 1Department of Physiology, College of Korean Medicine, Kyung Hee University, Seoul 130-701, Korea. hbae@khu.ac.kr
- 2Department of Korean Gynecology, College of Korean Medicine, Kyung Hee University, Seoul 130-701, Korea.
- 3Acupuncture and Meridian Science Research Center, Kyung Hee University, Seoul 130-701, Korea.
- KMID: 2285477
- DOI: http://doi.org/10.4196/kjpp.2013.17.6.505
Abstract
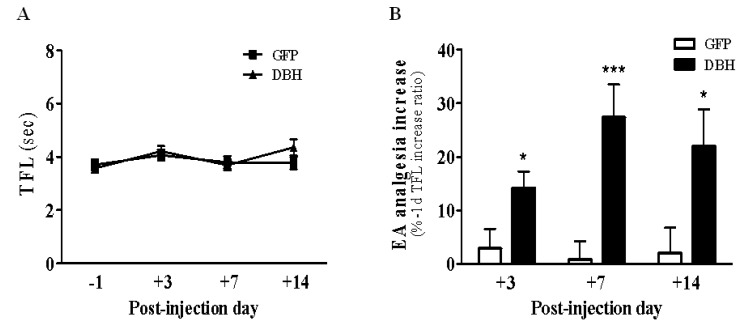
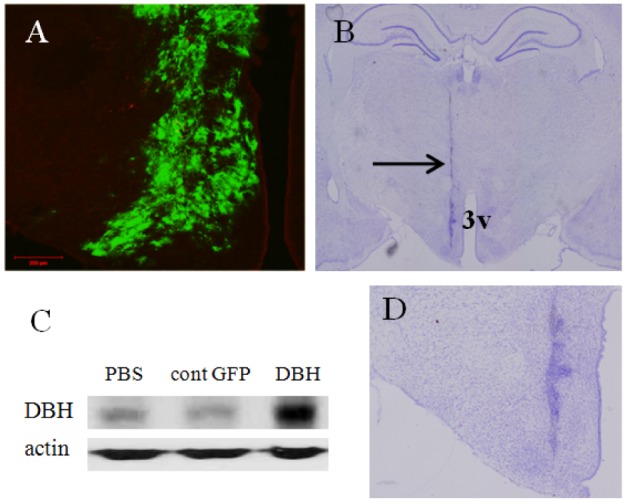
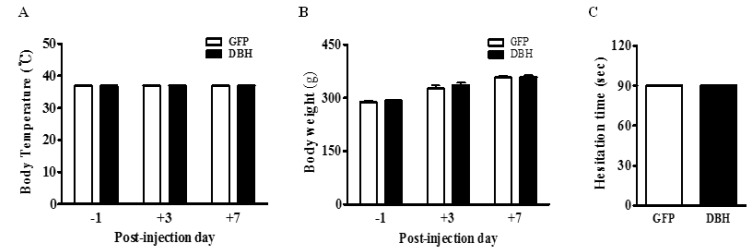
- Electroacupuncture (EA) is a modified form of acupuncture that utilizes electrical stimulation. We previously showed that EA stimulated rats were divided into responders that were sensitive to EA and non-responders that were insensitive to EA based on the tail flick latency (TFL) test. The dopamine beta-hydroxylase (DBH) gene was more abundantly expressed in the hypothalamus of responder rats than non-responder rats. To determine whether overexpression of DBH gene expression in the hypothalamus modulate EA analgesia, we constructed a DBH encoding adenovirus and which was then injected into the hypothalamus of SD rats. Microinjection of DBH or control GFP virus into the hypothalamus had no changes on the basal pain threshold measured by a TFL test without EA treatment. However, the analgesic effect of EA was significantly enhanced from seven days after microinjection of the DBH virus, but not after injection of the control GFP virus. DBH expression was significantly higher in the hypothalamus of DBH virus injected rat than control GFP virus or PBS injected rats. Moreover, expression of the DBH gene did not affect the body core temperature, body weight, motor function or learning and memory ability. Although the functional role of DBH in the hypothalamus in the analgesic effect of EA remains unclear, our findings suggest that expression of the DBH gene in the hypothalamus promotes EA analgesia without obvious side-effects.
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Acupuncture Stimulation at Different Acupoints on Formalin-Induced Pain in Rats
Kyung Ha Chang, Sun Joon Bai, Hyejung Lee, Bae Hwan Lee
Korean J Physiol Pharmacol. 2014;18(2):121-127. doi: 10.4196/kjpp.2014.18.2.121.
Reference
-
1. Lee H, Lee JY, Kim YJ, Kim S, Yin C, Khil JH, Kwon K, Choi SM, Lee H, Park HJ. Acupuncture for symptom management of rheumatoid arthritis: a pilot study. Clin Rheumatol. 2008; 27:641–645. PMID: 18193382.
Article2. Lin JG, Lo MW, Wen YR, Hsieh CL, Tsai SK, Sun WZ. The effect of high and low frequency electroacupuncture in pain after lower abdominal surgery. Pain. 2002; 99:509–514. PMID: 12406527.
Article3. Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003; 26:17–22. PMID: 12495858.
Article4. Baek YH, Choi DY, Yang HI, Park DS. Analgesic effect of electroacupuncture on inflammatory pain in the rat model of collagen-induced arthritis: mediation by cholinergic and serotonergic receptors. Brain Res. 2005; 1057:181–185. PMID: 16139820.
Article5. Chang FC, Tsai HY, Yu MC, Yi PL, Lin JG. The central serotonergic system mediates the analgesic effect of electroacupuncture on ZUSANLI (ST36) acupoints. J Biomed Sci. 2004; 11:179–185. PMID: 14966368.6. Kim SK, Park JH, Bae SJ, Kim JH, Hwang BG, Min BI, Park DS, Na HS. Effects of electroacupuncture on cold allodynia in a rat model of neuropathic pain: mediation by spinal adrenergic and serotonergic receptors. Exp Neurol. 2005; 195:430–436. PMID: 16054138.
Article7. Kanai S, Taniguchi N, Kanda K, Higashino H. Study of acupuncture stimulation on experimental osteopenia. Orient Pharm Exp Med. 2006; 6:79–85.8. Takeshige C, Sato T, Mera T, Hisamitsu T, Fang J. Descending pain inhibitory system involved in acupuncture analgesia. Brain Res Bull. 1992; 29:617–634. PMID: 1422859.
Article9. Ulett GA, Han S, Han JS. Electroacupuncture: mechanisms and clinical application. Biol Psychiatry. 1998; 44:129–138. PMID: 9646895.
Article10. Murotani T, Ishizuka T, Nakazawa H, Wang X, Mori K, Sasaki K, Ishida T, Yamatodani A. Possible involvement of histamine, dopamine, and noradrenalin in the periaqueductal gray in electroacupuncture pain relief. Brain Res. 2010; 1306:62–68. PMID: 19819232.
Article11. Lee G, Rho S, Shin M, Hong M, Min Bi, Bae H. The association of cholecystokinin-A receptor expression with the responsiveness of electroacupuncture analgesic effects in rat. Neurosci Lett. 2002; 325:17–20. PMID: 12023057.
Article12. Sur YC, Rho SW, Lee GS, Ko EJ, Hong MC, Shin MK, Min BI, Bae HS. Gene expression profile of the responder vs. the non-responder to the acupuncture mediated analgesic effects. Korean J Orient Physiol Pathol. 2003; 17:633–642.13. Zhou XJ, Yang J, Yan FL, Wang DX, Li XY, Fan XQ, Hao F, Yan XQ, Li XP, Li H, Liu WY, Lin BC. Norepinephrine plays an important role in antinociceptive modulation of hypothalamic paraventricular nucleus in the rat. Int J Neurosci. 2010; 120:428–438. PMID: 20504214.
Article14. Zhang C, Davies MF, Guo TZ, Maze M. The analgesic action of nitrous oxide is dependent on the release of norepinephrine in the dorsal horn of the spinal cord. Anesthesiology. 1999; 91:1401–1407. PMID: 10551592.
Article15. Monasky MS, Zinsmeister AR, Stevens CW, Yaksh TL. Interaction of intrathecal morphine and ST-91 on antinociception in the rat: dose-response analysis, antagonism and clearance. J Pharmacol Exp Ther. 1990; 254:383–392. PMID: 1974633.16. He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998; 95:2509–2514. PMID: 9482916.
Article17. Ko ES, Kim SK, Kim JT, Lee G, Han JB, Rho SW, Hong MC, Bae H, Min BI. The difference in mRNA expressions of hypothalamic CCK and CCK-A and -B receptors between responder and non-responder rats to high frequency electroacupuncture analgesia. Peptides. 2006; 27:1841–1845. PMID: 16472889.
Article18. Lee GS, Han JB, Shin MK, Hong MC, Kim SW, Min BI, Bae H. Enhancement of electroacupuncture-induced analgesic effect in cholecystokinin-A receptor deficient rats. Brain Res Bull. 2003; 62:161–164. PMID: 14638390.
Article19. Shim I, Ha Y, Chung JY, Lee HJ, Yang KH, Chang JW. Association of learning and memory impairments with changes in the septohippocampal cholinergic system in rats with kaolin-induced hydrocephalus. Neurosurgery. 2003; 53:416–425. discussion 425. PMID: 12925261.
Article20. Kim SK, Park JY, Koo BH, Lee JH, Kim HS, Choi WK, Shim I, Lee H, Hong MC, Shin MK, Min BI, Bae H. Adenoviral gene transfer of acetylcholinesterase T subunit in the hypothalamus potentiates electroacupuncture analgesia in rats. Genes Brain Behav. 2009; 8:174–180. PMID: 19077179.
Article21. Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002; 136:374–383. PMID: 11874310.
Article22. Kim JH, Min BI, Schmidt D, Lee HJ, Park DS. The difference between electroacupuncture only and electroacupuncture with manipulation on analgesia in rats. Neurosci Lett. 2000; 279:149–152. PMID: 10688051.
Article24. Zhang RX, Lao L, Wang L, Liu B, Wang X, Ren K, Berman BM. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res. 2004; 1020:12–17. PMID: 15312782.
Article25. Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc Natl Acad Sci U S A. 1999; 96:7744–7751. PMID: 10393892.
Article26. Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KV, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov A, Hruby VJ, Grisel JE, Fillingim RB. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A. 2003; 100:4867–4872. PMID: 12663858.
Article27. Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003; 299:1240–1243. PMID: 12595695.28. Dou C, Lee J, Liu B, Liu F, Massague J, Xuan S, Lai E. BF-1 interferes with transforming growth factor beta signaling by associating with Smad partners. Mol Cell Biol. 2000; 20:6201–6211. PMID: 10938097.29. Massoulié J, Anselmet A, Bon S, Krejci E, Legay C, Morel N, Simon S. The polymorphism of acetylcholinesterase: post-translational processing, quaternary associations and localization. Chem Biol Interact. 1999; 119-120:29–42. PMID: 10421436.
Article30. Gray J, Haran MM, Schneider K, Vesce S, Ray AM, Owen D, White IR, Cutler P, Davis JB. Evidence that inhibition of cathepsin-B contributes to the neuroprotective properties of caspase inhibitor Tyr-Val-Ala-Asp-chloromethyl ketone. J Biol Chem. 2001; 276:32750–32755. PMID: 11427531.
Article31. Tanemura K, Murayama M, Akagi T, Hashikawa T, Tominaga T, Ichikawa M, Yamaguchi H, Takashima A. Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J Neurosci. 2002; 22:133–141. PMID: 11756496.
Article32. Jasmin L, Tien D, Weinshenker D, Palmiter RD, Green PG, Janni G, Ohara PT. The NK1 receptor mediates both the hyperalgesia and the resistance to morphine in mice lacking noradrenaline. Proc Natl Acad Sci U S A. 2002; 99:1029–1034. PMID: 11805341.
Article33. Takeshige C. Synaptic transmission in acupuncture analgesia. Tokyo: Showa University School of Medicine;1992.34. Han JS. The neurochemical basis of pain relief by acupuncture. Beijing: Chinese Medical Science and Techonology Press;1987.35. Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001; 53:597–652. PMID: 11734620.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-stress effects of ginseng via down-regulation of tyrosine hydroxylase (TH) and dopamine beta-hydroxylase (DBH) gene expression in immobilization-stressed rats and PC12 cells
- Analgesic Mechanism of Electroacupuncture in an Arthritic Pain Model of Rats: A Neurotransmitter Study
- Effective Acupoint of Electroacupuncture on Ankle-sprained Pain in Rats
- In Vitro Release of L-dopa and Dopamine by Genetically Modified Fibroblasts of the Rat
- Dopaminergic Neurons in the Diencephalon of Striped Field Mouse[Apodemus agrarius coreae]