Korean J Physiol Pharmacol.
2013 Aug;17(4):331-338. 10.4196/kjpp.2013.17.4.331.
Neuroprotective Effects of AMP-Activated Protein Kinase on Scopolamine Induced Memory Impairment
- Affiliations
-
- 1College of Korean Medicine, Kyung Hee University, Seoul 130-701, Korea. hbae@khu.ac.kr
- 2School of Medicine, Kyung Hee University, Seoul 130-701, Korea.
- 3Institute of Oriental Medicine, Kyung Hee University, Seoul 130-701, Korea.
- KMID: 2285468
- DOI: http://doi.org/10.4196/kjpp.2013.17.4.331
Abstract
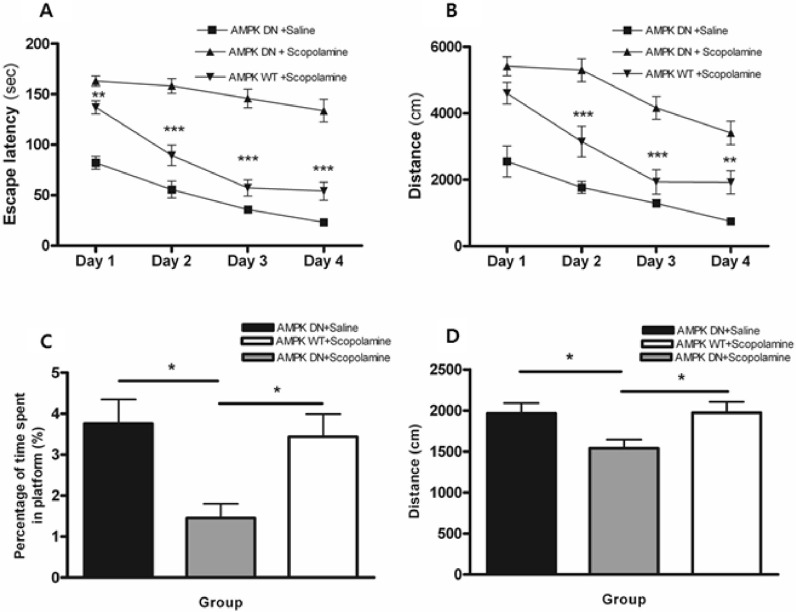
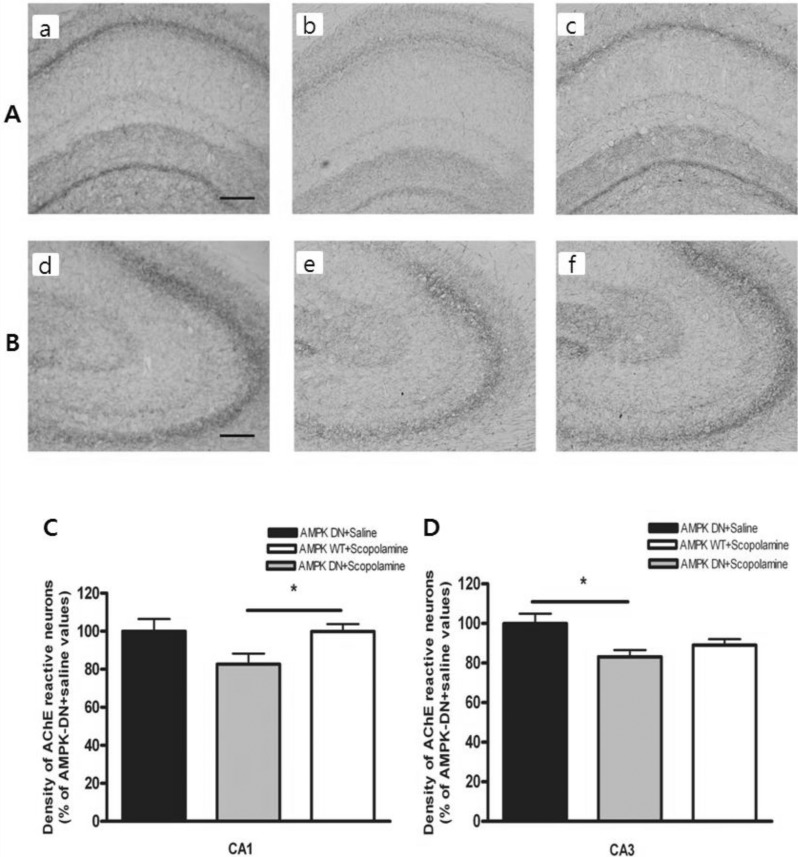
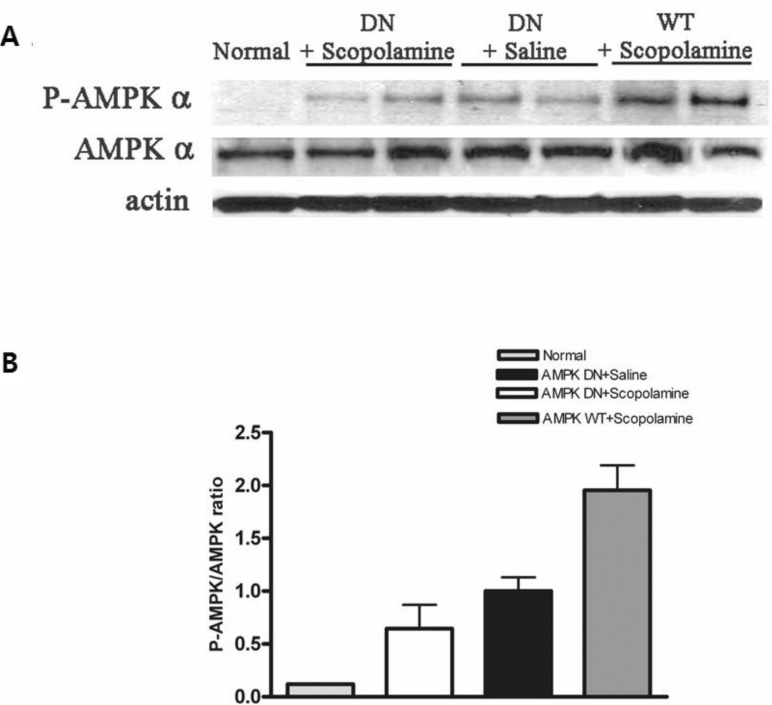
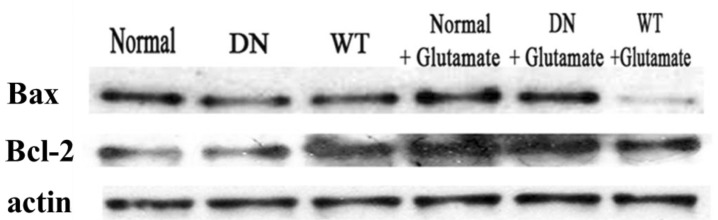
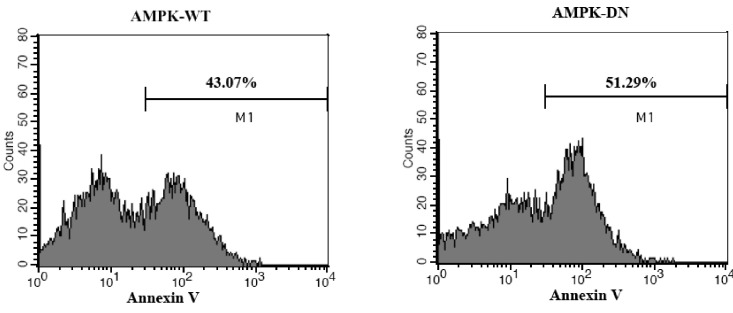
- AMP-activated protein kinase (AMPK), an important regulator of energy metabolism, is activated in response to cellular stress when intracellular levels of AMP increase. We investigated the neuroprotective effects of AMPK against scopolamine-induced memory impairment in vivo and glutamate-induced cytotoxicity in vitro. An adenovirus expressing AMPK wild type alpha subunit (WT) or a dominant negative form (DN) was injected into the hippocampus of rats using a stereotaxic apparatus. The AMPK WT-injected rats showed significant reversal of the scopolamine induced cognitive deficit as evaluated by escape latency in the Morris water maze. In addition, they showed enhanced acetylcholinesterase (AChE)-reactive neurons in the hippocampus, implying increased cholinergic activity in response to AMPK. We also studied the cellular mechanism by which AMPK protects against glutamate-induced cell death in primary cultured rat hippocampal neurons. We further demonstrated that AMPK WT-infected cells increased cell viability and reduced Annexin V positive hippocampal neurons. Western blot analysis indicated that AMPK WT-infected cells reduced the expression of Bax and had no effects on Bcl-2, which resulted in a decreased Bax/Bcl-2 ratio. These data suggest that AMPK is a useful cognitive impairment treatment target, and that its beneficial effects are mediated via the protective capacity of hippocampal neurons.
Keyword
MeSH Terms
-
Acetylcholinesterase
Adenoviridae
AMP-Activated Protein Kinases
Animals
Annexin A5
Apoptosis
Blotting, Western
Cell Death
Cell Survival
Energy Metabolism
Hippocampus
Memory
Neurons
Neuroprotective Agents
Rats
Scopolamine Hydrobromide
United Nations
AMP-Activated Protein Kinases
Acetylcholinesterase
Annexin A5
Neuroprotective Agents
Scopolamine Hydrobromide
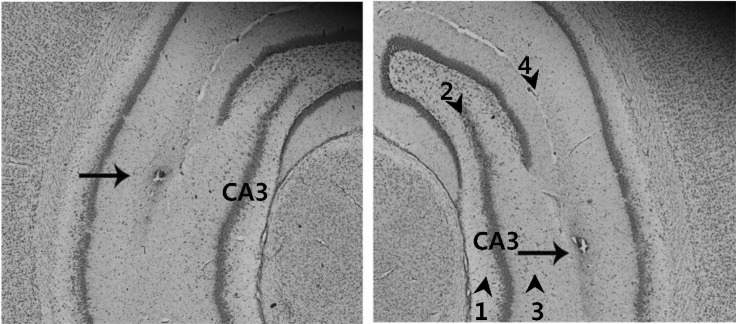
Figure
Cited by 3 articles
-
Ameliorative Effect of a Selective Endothelin ETA Receptor Antagonist in Rat Model of L-Methionine-induced Vascular Dementia
Gautamjeet S Mangat, Amteshwar S Jaggi, Nirmal Singh
Korean J Physiol Pharmacol. 2014;18(3):201-209. doi: 10.4196/kjpp.2014.18.3.201.EGCG Blocked Phenylephrin-Induced Hypertrophy in H9C2 Cardiomyocytes, by Activating AMPK-Dependent Pathway
Yi Cai, Li Zhao, Yuan Qin, Xiao-Qian Wu
Korean J Physiol Pharmacol. 2015;19(3):203-210. doi: 10.4196/kjpp.2015.19.3.203.Resveratrol attenuates lipopolysaccharide-induced dysfunction of blood-brain barrier in endothelial cells via AMPK activation
Min Hu, Bo Liu
Korean J Physiol Pharmacol. 2016;20(4):325-332. doi: 10.4196/kjpp.2016.20.4.325.
Reference
-
1. Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008; 28:2278–2287. PMID: 19046371.
Article2. Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998; 67:821–855. PMID: 9759505.
Article3. Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003; 144:5179–5183. PMID: 12960015.
Article4. Hardie DG. The AMP-activated protein kinase pathway-new players upstream and downstream. J Cell Sci. 2004; 117:5479–5487. PMID: 15509864.5. Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferré P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000; 20:6704–6711. PMID: 10958668.
Article6. Johnson LN, Noble ME, Owen DJ. Active and inactive protein kinases: structural basis for regulation. Cell. 1996; 85:149–158. PMID: 8612268.
Article7. Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J. 2000; 345:437–443. PMID: 10642499.
Article8. Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001; 17:45–58. PMID: 11665862.
Article9. Witters LA, Kemp BE, Means AR. Chutes and ladders: the search for protein kinases that act on AMPK. Trends Biochem Sci. 2006; 31:13–16. PMID: 16356723.
Article10. Zhao Y, Shen J, Su H, Li B, Xing D, Du L. Chronic corticosterone injections induce a decrease of ATP levels and sustained activation of AMP-activated protein kinase in hippocampal tissues of male mice. Brain Res. 2008; 1191:148–156. PMID: 18164281.
Article11. Gadalla AE, Pearson T, Currie AJ, Dale N, Hawley SA, Sheehan M, Hirst W, Michel AD, Randall A, Hardie DG, Frenguelli BG. AICA riboside both activates AMP-activated protein kinase and competes with adenosine for the nucleoside transporter in the CA1 region of the rat hippocampus. J Neurochem. 2004; 88:1272–1282. PMID: 15009683.
Article12. Föller M, Sopjani M, Koka S, Gu S, Mahmud H, Wang K, Floride E, Schleicher E, Schulz E, Münzel T, Lang F. Regulation of erythrocyte survival by AMP-activated protein kinase. FASEB J. 2009; 23:1072–1080. PMID: 19050047.
Article13. McGee SL, Hargreaves M. AMPK and transcriptional regulation. Front Biosci. 2008; 13:3022–3033. PMID: 17981775.
Article14. Lee B, Sur B, Shim I, Lee H, Hahm DH. Phellodendron amurense and its major alkaloid compound, berberine ameliorates scopolamine-induced neuronal impairment and memory dysfunction in rats. Korean J Physiol Pharmacol. 2012; 16:79–89. PMID: 22563252.15. Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging. 2005; 26:939–946. PMID: 15718053.
Article16. Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev. 2010; 34:1307–1350. PMID: 20398692.
Article17. Goverdhan P, Sravanthi A, Mamatha T. Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int J Alzheimers Dis. 2012; 2012:974013. PMID: 22536538.
Article18. Fan Y, Hu J, Li J, Yang Z, Xin X, Wang J, Ding J, Geng M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci Lett. 2005; 374:222–226. PMID: 15663967.
Article19. Ray CA, Blin J, Chase TN, Piercey MF. Effects of cholinergic agonists on regional brain energy metabolism in the scopolamine-treated rat. Neuropharmacology. 1992; 31:1193–1199. PMID: 1475026.
Article20. Piercey MF, Vogelsang GD, Franklin SR, Tang AH. Reversal of scopolamine-induced amnesia and alterations in energy metabolism by the nootropic piracetam: implications regarding identification of brain structures involved in consolidation of memory traces. Brain Res. 1987; 424:1–9. PMID: 3690290.
Article21. Schubert D, Piasecki D. Oxidative glutamate toxicity can be a component of the excitotoxicity cascade. J Neurosci. 2001; 21:7455–7462. PMID: 11567035.
Article22. Albright TD, Jessell TM, Kandel ER, Posner MI. Neural science: a century of progress and the mysteries that remain. Cell. 2000; 100(Suppl):S1–S55. PMID: 10698132.
Article23. Lee S, Kim Y, Li E, Park S. Ghrelin protects spinal cord motoneurons against chronic glutamate excitotoxicity by inhibiting microglial activation. Korean J Physiol Pharmacol. 2012; 16:43–48. PMID: 22416219.
Article24. Leon R, Wu H, Jin Y, Wei J, Buddhala C, Prentice H, Wu JY. Protective function of taurine in glutamate-induced apoptosis in cultured neurons. J Neurosci Res. 2009; 87:1185–1194. PMID: 18951478.
Article25. Mark LP, Prost RW, Ulmer JL, Smith MM, Daniels DL, Strottmann JM, Brown WD, Hacein-Bey L. Pictorial review of glutamate excitotoxicity: fundamental concepts for neuroimaging. AJNR Am J Neuroradiol. 2001; 22:1813–1824. PMID: 11733308.26. McConkey DJ. Biochemical determinants of apoptosis and necrosis. Toxicol Lett. 1998; 99:157–168. PMID: 9862281.
Article27. Lee JH, Choi S, Kim JH, Kim JK, Kim JI, Nah SY. Effects of ginsenosides on carbachol-stimulated formation of inositol phosphates in rat cortical cell cultures. Neurochem Res. 2003; 28:1307–1313. PMID: 12938851.28. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11:47–60. PMID: 6471907.
Article29. Beere HM. Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest. 2005; 115:2633–2639. PMID: 16200196.
Article30. Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999; 13:1899–1911. PMID: 10444588.
Article31. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995; 184:39–51. PMID: 7622868.
Article32. Hardie DG, Frenguelli BG. A neural protection racket: AMPK and the GABA(B) receptor. Neuron. 2007; 53:159–162. PMID: 17224398.
Article33. Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem. 1999; 72:1707–1716. PMID: 10098881.
Article34. Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest. 2000; 105:1711–1721. PMID: 10862786.
Article35. Dagon Y, Avraham Y, Magen I, Gertler A, Ben-Hur T, Berry EM. Nutritional status, cognition, and survival: a new role for leptin and AMP kinase. J Biol Chem. 2005; 280:42142–42148. PMID: 16203737.36. Spasić MR, Callaerts P, Norga KK. AMP-activated protein kinase (AMPK) molecular crossroad for metabolic control and survival of neurons. Neuroscientist. 2009; 15:309–316. PMID: 19359670.
Article37. Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, Iemata M, Warren N, Couve A, Calver A, Horvath Z, Freeman K, Carling D, Huang L, Gonzales C, Cooper E, Smart TG, Pangalos MN, Moss SJ. Phospho-dependent functional modulation of GABA (B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007; 53:233–247. PMID: 17224405.38. Yoon H, Oh YT, Lee JY, Choi JH, Lee JH, Baik HH, Kim SS, Choe W, Yoon KS, Ha J, Kang I. Activation of AMP-activated protein kinase by kainic acid mediates brain-derived neurotrophic factor expression through a NF-kappaB dependent mechanism in C6 glioma cells. Biochem Biophys Res Commun. 2008; 371:495–500. PMID: 18445478.
Article39. Wang W, Fan J, Yang X, Fürer-Galban S, Lopez de Silanes I, von Kobbe C, Guo J, Georas SN, Foufelle F, Hardie DG, Carling D, Gorospe M. AMP-activated kinase regulates cytoplasmic HuR. Mol Cell Biol. 2002; 22:3425–3436. PMID: 11971974.
Article40. Wang W, Yang X, López de Silanes I, Carling D, Gorospe M. Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J Biol Chem. 2003; 278:27016–27023. PMID: 12730239.
Article41. Dagon Y, Avraham Y, Berry EM. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2alpha in adipocytes. Biochem Biophys Res Commun. 2006; 340:43–47. PMID: 16377306.42. Jung JE, Lee J, Ha J, Kim SS, Cho YH, Baik HH, Kang I. 5-Aminoimidazole-4-carboxamide-ribonucleoside enhances oxidative stress-induced apoptosis through activation of nuclear factor-kappaB in mouse Neuro 2a neuroblastoma cells. Neurosci Lett. 2004; 354:197–200. PMID: 14700730.43. Kefas BA, Cai Y, Kerckhofs K, Ling Z, Martens G, Heimberg H, Pipeleers D, Van de Casteele M. Metformin-induced stimulation of AMP-activated protein kinase in beta-cells impairs their glucose responsiveness and can lead to apoptosis. Biochem Pharmacol. 2004; 68:409–416. PMID: 15242807.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Glycyrrhizic Acid on Scopolamine-Induced Cognitive Impairment in Mice
- Letter to the Editor: 17Beta-estradiol Stimulates Glucose Uptake Through Estrogen Receptor and AMP-activated Protein Kinase Activation in C2C12 Myotubes (Korean J Obes 2016;25:190-6)
- Neurobiological Mechanism of Memory
- Phellodendron amurense and Its Major Alkaloid Compound, Berberine Ameliorates Scopolamine-Induced Neuronal Impairment and Memory Dysfunction in Rats
- Fraxetin Induces Heme Oxygenase-1 Expression by Activation of Akt/Nrf2 or AMP-activated Protein Kinase α/Nrf2 Pathway in HaCaT Cells