Korean J Physiol Pharmacol.
2012 Dec;16(6):463-468. 10.4196/kjpp.2012.16.6.463.
Effect of PRX-1 Downregulation in the Type 1 Diabetes Microenvironment
- Affiliations
-
- 1Laboratory of Host Defense Modulation, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. khwang@cau.ac.kr
- 2Department of Pharmacognosy, College of Pharmacy, Dankook University, Chungnam 330-714, Korea.
- KMID: 2285456
- DOI: http://doi.org/10.4196/kjpp.2012.16.6.463
Abstract
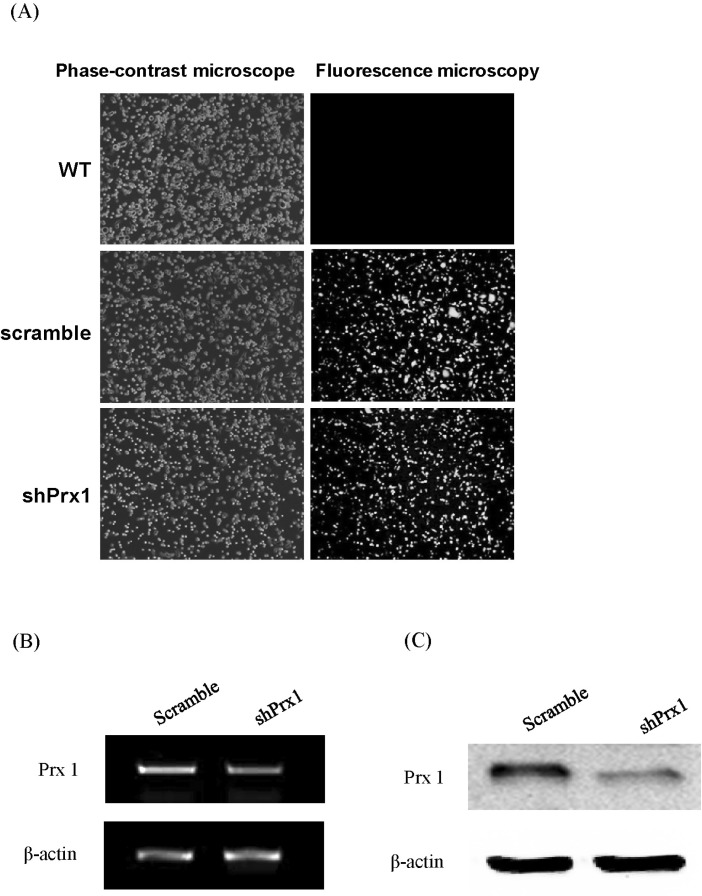
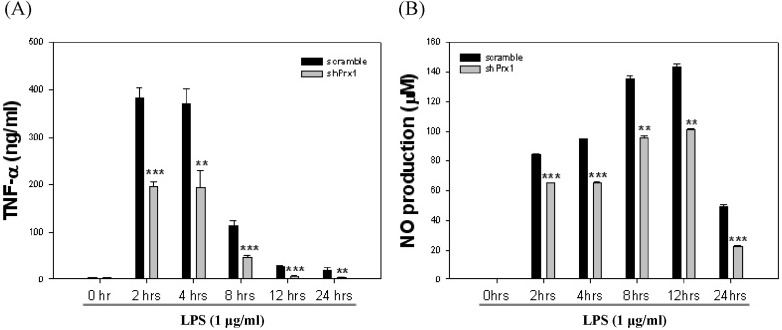
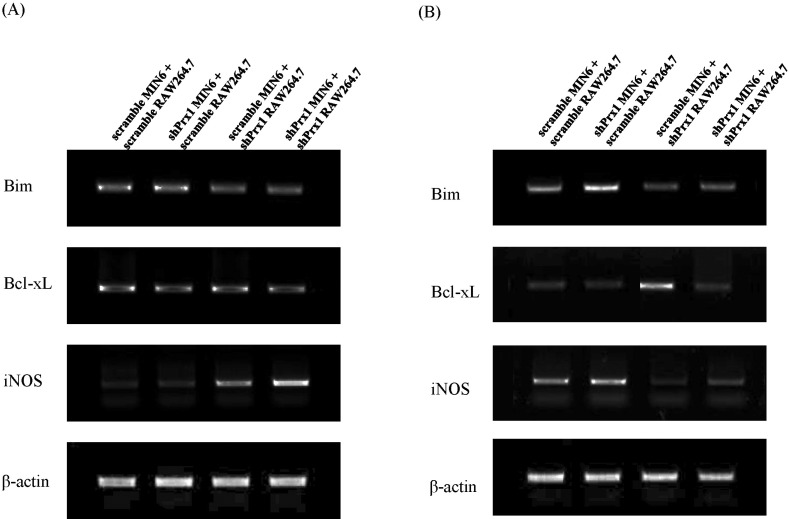
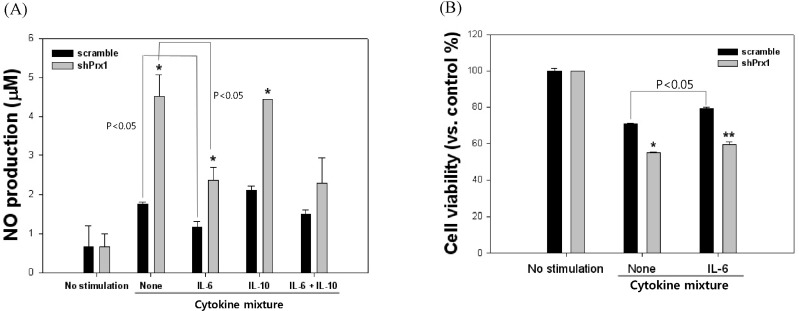
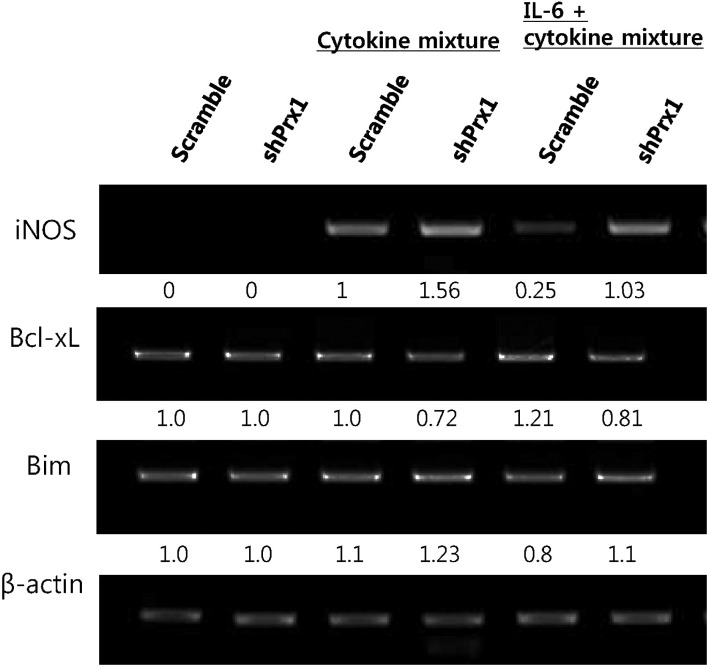
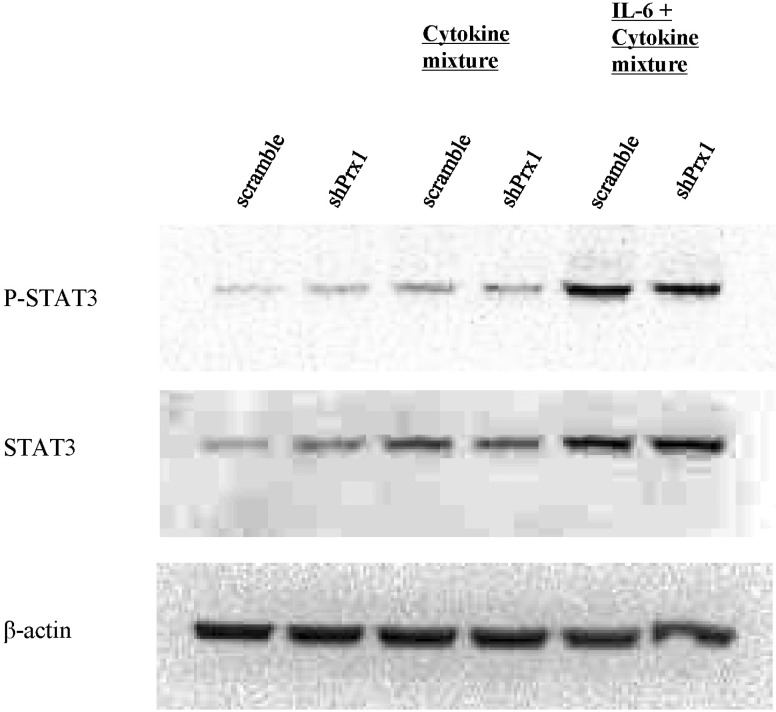
- Type 1 diabetes (T1D) is caused by dysregulation of the immune system in the pancreatic islets, which eventually leads to insulin-producing pancreatic beta-cell death and destabilization of glucose homeostasis. One of the major characteristics of T1D pathogenesis is the production of inflammatory mediators by macrophages that result in destruction or damage of pancreatic beta-cells. In this study the inflammatory microenvironment of T1D was simulated with RAW264.7 cells and MIN6 cells, acting as macrophages and pancreatic beta-cells respectably. In this setting, peroxiredoxin-1, an anti-oxidant enzyme was knocked down to observe its functions in the pathogenesis of T1D. RAW264.7 cells were primed with lipopolysaccharide and co-cultured with MIN6 cells while PRX-1 was knocked down in one or both cell types. Our results suggest that hindrance of PRX-1 activity or the deficiency of this enzyme in inflammatory conditions negatively affects pancreatic beta-cell survival. The observed decrease in viability of MIN6 cells seems to be caused by nitric oxide production. Additionally, it seems that PRX-1 affects previously reported protective activity of IL-6 in pancreatic beta cells as well. These results signify new, undiscovered roles for PRX-1 in inflammatory conditions and may contribute toward our understanding of autoimmunity.
MeSH Terms
Figure
Reference
-
1. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005; 115:1111–1119. PMID: 15864338.
Article2. Rabinovitch A, Suarez-Pinzon WL. Roles of cytokines in the pathogenesis and therapy of type 1 diabetes. Cell Biochem Biophys. 2007; 48:159–163. PMID: 17709885.
Article3. Yoon JW, Jun HS. Cellular and molecular roles of beta cell autoantigens, macrophages and T cells in the pathogenesis of autoimmune diabetes. Arch Pharm Res. 1999; 22:437–447. PMID: 10549569.4. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005; 4:281–286. PMID: 16101534.
Article5. Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki JI, Oka Y. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia. 1993; 36:1139–1145. PMID: 8270128.
Article6. Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005; 38:1543–1552. PMID: 15917183.
Article7. Bast A, Wolf G, Oberbäumer I, Walther R. Oxidative and nitrosative stress induces peroxiredoxins in pancreatic beta cells. Diabetologia. 2002; 45:867–876. PMID: 12107731.
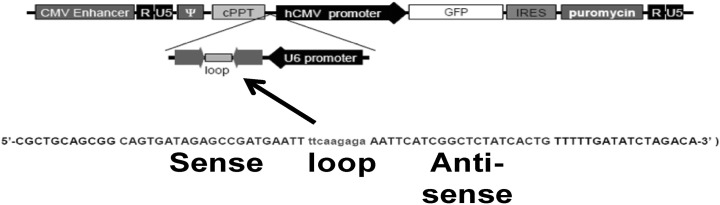
Article8. Tae Lim Y, Sup Song D, Joon Won T, Lee YJ, Yoo JS, Eun Hyung K, Won Yoon J, Park SY, Woo Hwang K. Peroxiredoxin-1, a possible target in modulating inflammatory cytokine production in macrophage like cell line RAW264.7. Microbiol Immunol. 2012; 56:411–419. PMID: 22486405.
Article9. Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011; 22:83–89. PMID: 21377916.
Article10. Holohan C, Szegezdi E, Ritter T, O'Brien T, Samali A. Cytokine-induced beta-cell apoptosis is NO-dependent, mitochondria-mediated and inhibited by BCL-XL. J Cell Mol Med. 2008; 12:591–606. PMID: 18081694.11. Wolf G, Aumann N, Michalska M, Bast A, Sonnemann J, Beck JF, Lendeckel U, Newsholme P, Walther R. Peroxiredoxin III protects pancreatic β cells from apoptosis. J Endocrinol. 2010; 207:163–175. PMID: 20807727.
Article12. Park H, Ahn Y, Park CK, Chung HY, Park Y. Interleukin-6 protects MIN6 beta cells from cytokine-induced apoptosis. Ann N Y Acad Sci. 2003; 1005:242–249. PMID: 14679069.13. Choi SE, Choi KM, Yoon IH, Shin JY, Kim JS, Park WY, Han DJ, Kim SC, Ahn C, Kim JY, Hwang ES, Cha CY, Szot GL, Yoon KH, Park CG. IL-6 protects pancreatic islet beta cells from pro-inflammatory cytokines-induced cell death and functional impairment in vitro and in vivo. Transpl Immunol. 2004; 13:43–53. PMID: 15203128.14. Campbell IL, Cutri A, Wilson A, Harrison LC. Evidence for IL-6 production by and effects on the pancreatic beta-cell. J Immunol. 1989; 143:1188–1191. PMID: 2501390.15. Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011; 31:379–446. PMID: 22142165.
Article16. Wogensen L, Lee MS, Sarvetnick N. Production of interleukin 10 by islet cells accelerates immune-mediated destruction of beta cells in nonobese diabetic mice. J Exp Med. 1994; 179:1379–1384. PMID: 8145050.
Article17. Liu A, Liu Y, Li PK, Li C, Lin J. LLL12 inhibits endogenous and exogenous interleukin-6-induced STAT3 phosphorylation in human pancreatic cancer cells. Anticancer Res. 2011; 31:2029–2035. PMID: 21737619.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Peroxiredoxin III on Human HeLa Cell Proliferation
- Expression of Peroxiredoxin I and II in Neonatal and Adult Rat Lung Exposed to Hyperoxia
- The Inhibition of Human Telomerase Reverse Transcriptase Expression by Peroxiredoxin I and c-Myc in Prostatic Cancer Cells
- Synergistic effect of peroxiredoxin II antisense on cisplatin-induced cell death
- Glial and Vascular Cell Regulation of the Blood-Brain Barrier in Diabetes