Korean J Physiol Pharmacol.
2012 Dec;16(6):423-429. 10.4196/kjpp.2012.16.6.423.
Oxygen/Glucose Deprivation and Reperfusion Cause Modifications of Postsynaptic Morphology and Activity in the CA3 Area of Organotypic Hippocampal Slice Cultures
- Affiliations
-
- 1Department of Pharmacology and Ewha Medical Research Institute, Ewha Womans University School of Medicine, Seoul 158-710, Korea. kelee@ewha.ac.kr
- KMID: 2285451
- DOI: http://doi.org/10.4196/kjpp.2012.16.6.423
Abstract
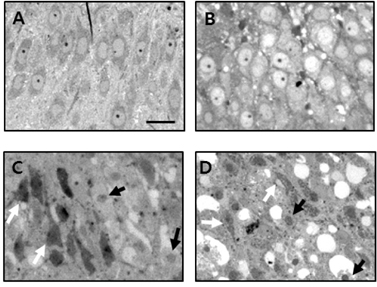
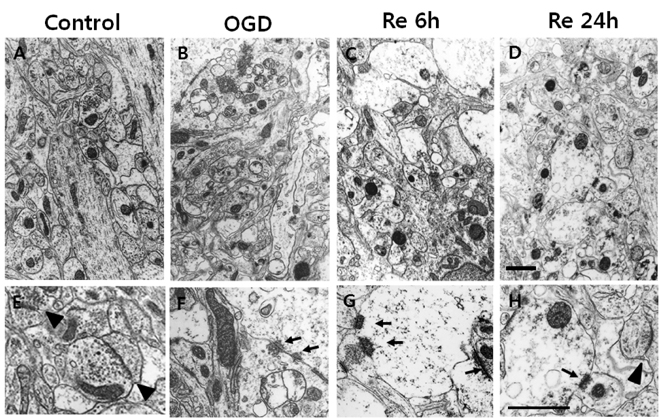
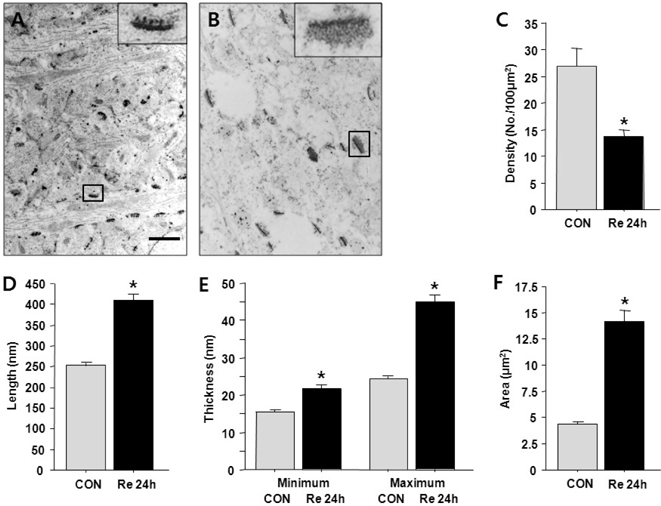
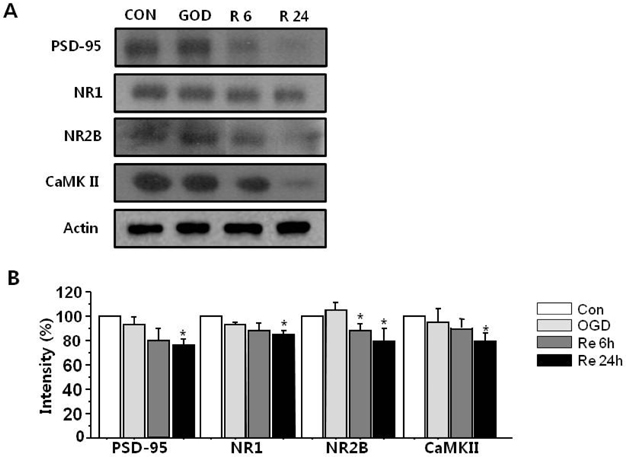
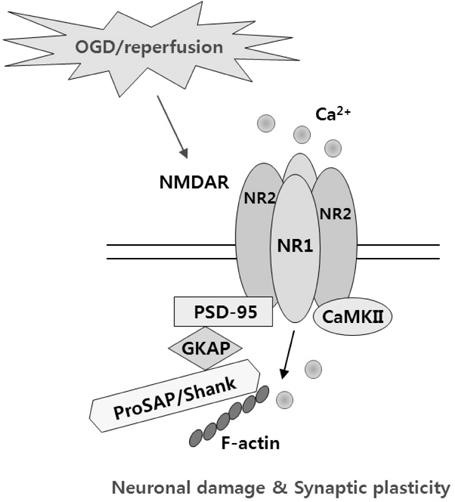
- Brain ischemia leads to overstimulation of N-methyl-D-aspartate (NMDA) receptors, referred as excitotoxicity, which mediates neuronal cell death. However, less attention has been paid to changes in synaptic activity and morphology that could have an important impact on cell function and survival following ischemic insult. In this study, we investigated the effects of reperfusion after oxygen/glucose deprivation (OGD) not only upon neuronal cell death, but also on ultrastructural and biochemical characteristics of postsynaptic density (PSD) protein, in the stratum lucidum of the CA3 area in organotypic hippocampal slice cultures. After OGD/reperfusion, neurons were found to be damaged; the organelles such as mitochondria, endoplasmic reticulum, dendrites, and synaptic terminals were swollen; and the PSD became thicker and irregular. Ethanolic phosphotungstic acid staining showed that the density of PSD was significantly decreased, and the thickness and length of the PSD were significantly increased in the OGD/reperfusion group compared to the control. The levels of PSD proteins, including PSD-95, NMDA receptor 1, NMDA receptor 2B, and calcium/calmodulin-dependent protein kinase II, were significantly decreased following OGD/reperfusion. These results suggest that OGD/reperfusion induces significant modifications to PSDs in the CA3 area of organotypic hippocampal slice cultures, both morphologically and biochemically, and this may contribute to neuronal cell death and synaptic dysfunction after OGD/reperfusion.
Keyword
MeSH Terms
-
Brain Ischemia
Cell Death
Dendrites
Endoplasmic Reticulum
Ethanol
Mitochondria
N-Methylaspartate
Neurons
Organelles
Phosphotungstic Acid
Post-Synaptic Density
Presynaptic Terminals
Protein Kinases
Proteins
Receptors, N-Methyl-D-Aspartate
Reperfusion
Ethanol
N-Methylaspartate
Phosphotungstic Acid
Protein Kinases
Proteins
Receptors, N-Methyl-D-Aspartate
Figure
Cited by 2 articles
-
Airway Smooth Muscle Sensitivity to Methacholine in Precision-Cut Lung Slices (PCLS) from Ovalbumin-induced Asthmatic Mice
Hae Jin Kim, Yeryung Kim, Su Jung Park, Boram Bae, Hye-Ryun Kang, Sang-Heon Cho, Hae Young Yoo, Joo Hyun Nam, Woo Kyung Kim, Sung Joon Kim
Korean J Physiol Pharmacol. 2015;19(1):65-71. doi: 10.4196/kjpp.2015.19.1.65.Neuroprotective effect of caffeic acid phenethyl ester in 3-nitropropionic acid-induced striatal neurotoxicity
Jia Bak, Hee Jung Kim, Seong Yun Kim, Yun-Sik Choi
Korean J Physiol Pharmacol. 2016;20(3):279-286. doi: 10.4196/kjpp.2016.20.3.279.
Reference
-
1. Arundine M, Chopra GK, Wrong A, Lei S, Aarts MM, MacDonald JF, Tymianski M. Enhanced vulnerability to NMDA toxicity in sublethal traumatic neuronal injury in vitro. J Neurotrauma. 2003. 20:1377–1395.2. Choi DW. Excitotoxic cell death. J Neurobiol. 1992. 23:1261–1276.3. Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994. 330:613–622.4. Lo HM, Hsu KL, Lin FY, Tseng YZ. Implications of prolonged pause in patients with chronic atrial fibrillation with mitral valve disease undergoing atrial compartment operation. J Formos Med Assoc. 2003. 102:762–767.5. Jourdain P, Nikonenko I, Alberi S, Muller D. Remodeling of hippocampal synaptic networks by a brief anoxia-hypoglycemia. J Neurosci. 2002. 22:3108–3116.6. Nikonenko A, Schmidt S, Skibo G, Brückner G, Schachner M. Tenascin-R-deficient mice show structural alterations of symmetric perisomatic synapses in the CA1 region of the hippocampus. J Comp Neurol. 2003. 456:338–349.7. Schmidt-Kastner R, Fliss H, Hakim AM. Subtle neuronal death in striatum after short forebrain ischemia in rats detected by in situ end-labeling for DNA damage. Stroke. 1997. 28:163–169.8. Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, Sheng M, Peng J. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006. 5:1158–1170.9. Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, Choudhary JS, Grant SG. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J Neurochem. 2006. 97:Suppl 1. 16–23.10. Kennedy MB. The postsynaptic density at glutamatergic synapses. Trends Neurosci. 1997. 20:264–268.11. Verpelli C, Schmeisser MJ, Sala C, Boeckers TM. Scaffold proteins at the postsynaptic density. Adv Exp Med Biol. 2012. 970:29–61.12. Aoki C, Miko I, Oviedo H, Mikeladze-Dvali T, Alexandre L, Sweeney N, Bredt DS. Electron microscopic immunocytochemical detection of PSD-95, PSD-93, SAP-102, and SAP-97 at postsynaptic, presynaptic, and nonsynaptic sites of adult and neonatal rat visual cortex. Synapse. 2001. 40:239–257.13. Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000. 27:57–69.14. Garner CC, Kindler S, Gundelfinger ED. Molecular determinants of presynaptic active zones. Curr Opin Neurobiol. 2000. 10:321–327.15. Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007. 17:343–352.16. Kennedy MB. Signal transduction molecules at the glutamatergic postsynaptic membrane. Brain Res Brain Res Rev. 1998. 26:243–257.17. Bredt DS. NO NMDA receptor activity. Nat Biotechnol. 1996. 14:944.18. Losi G, Prybylowski K, Fu Z, Luo J, Wenthold RJ, Vicini S. PSD-95 regulates NMDA receptors in developing cerebellar granule neurons of the rat. J Physiol. 2003. 548:21–29.19. Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001. 4:794–802.20. Townsend M, Yoshii A, Mishina M, Constantine-Paton M. Developmental loss of miniature N-methyl-D-aspartate receptor currents in NR2A knockout mice. Proc Natl Acad Sci U S A. 2003. 100:1340–1345.21. Choi DW. Bench to bedside: the glutamate connection. Science. 1992. 258:241–243.22. Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995. 269:1737–1740.23. Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996. 16:2157–2163.24. Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001. 11:327–335.25. Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005. 438:185–192.26. Köhr G. NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res. 2006. 326:439–446.27. Goldenring JR, McGuire JS Jr, DeLorenzo RJ. Identification of the major postsynaptic density protein as homologous with the major calmodulin-binding subunit of a calmodulin-dependent protein kinase. J Neurochem. 1984. 42:1077–1084.28. Suzuki T, Okumura-Noji K, Tanaka R, Tada T. Rapid translocation of cytosolic Ca2+/calmodulin-dependent protein kinase II into postsynaptic density after decapitation. J Neurochem. 1994. 63:1529–1537.29. Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999. 285:1870–1874.30. Okabe S. Molecular anatomy of the postsynaptic density. Mol Cell Neurosci. 2007. 34:503–518.31. Jung YJ, Park SJ, Park JS, Lee KE. Glucose/oxygen deprivation induces the alteration of synapsin I and phosphosynapsin. Brain Res. 2004. 996:47–54.32. Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991. 37:173–182.33. Ahlgren H, Henjum K, Ottersen OP, Rundén-Pran E. Validation of organotypical hippocampal slice cultures as an ex vivo model of brain ischemia: different roles of NMDA receptors in cell death signalling after exposure to NMDA or oxygen and glucose deprivation. Cell Tissue Res. 2011. 345:329–341.34. Bloom FE, Aghajanian GK. Fine structural and cytochemical analysis of the staining of synaptic junctions with phosphotungstic acid. J Ultrastruct Res. 1968. 22:361–375.35. Hu BR, Janelidze S, Ginsberg MD, Busto R, Perez-Pinzon M, Sick TJ, Siesjö BK, Liu CL. Protein aggregation after focal brain ischemia and reperfusion. J Cereb Blood Flow Metab. 2001. 21:865–875.36. Suh EC, Jung YJ, Kim YA, Park EM, Lee KE. A beta 25-35 induces presynaptic changes in organotypic hippocampal slice cultures. Neurotoxicology. 2008. 29:691–699.37. Kovalenko T, Osadchenko I, Nikonenko A, Lushnikova I, Voronin K, Nikonenko I, Muller D, Skibo G. Ischemia-induced modifications in hippocampal CA1 stratum radiatum excitatory synapses. Hippocampus. 2006. 16:814–825.38. Ruan YW, Han XJ, Shi ZS, Lei ZG, Xu ZC. Remodeling of synapses in the CA1 area of the hippocampus after transient global ischemia. Neuroscience. 2012. 218:268–277.39. Mori K, Togashi H, Ueno KI, Matsumoto M, Yoshioka M. Aminoguanidine prevented the impairment of learning behavior and hippocampal long-term potentiation following transient cerebral ischemia. Behav Brain Res. 2001. 120:159–168.40. Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979. 10:267–272.41. Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002. 298:846–850.42. Kirino T, Tsujita Y, Tamura A. Induced tolerance to ischemia in gerbil hippocampal neurons. J Cereb Blood Flow Metab. 1991. 11:299–307.43. Park SJ, Jung YJ, Kim YA, Lee-Kang JH, Lee KE. Glucose/oxygen deprivation and reperfusion upregulate SNAREs and complexin in organotypic hippocampal slice cultures. Neuropathology. 2008. 28:612–620.44. Kirino T, Robinson HP, Miwa A, Tamura A, Kawai N. Disturbance of membrane function preceding ischemic delayed neuronal death in the gerbil hippocampus. J Cereb Blood Flow Metab. 1992. 12:408–417.45. Pulsinelli WA, Levy DE, Duffy TE. Regional cerebral blood flow and glucose metabolism following transient forebrain ischemia. Ann Neurol. 1982. 11:499–502.46. Petito CK, Pulsinelli WA. Delayed neuronal recovery and neuronal death in rat hippocampus following severe cerebral ischemia: possible relationship to abnormalities in neuronal processes. J Cereb Blood Flow Metab. 1984. 4:194–205.47. Smith ML, Bendek G, Dahlgren N, Rosén I, Wieloch T, Siesjö BK. Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol Scand. 1984. 69:385–401.48. Leblond J, Krnjevic K. Hypoxic changes in hippocampal neurons. J Neurophysiol. 1989. 62:1–14.49. Lobner D, Lipton P. Intracellular calcium levels and calcium fluxes in the CA1 region of the rat hippocampal slice during in vitro ischemia: relationship to electrophysiological cell damage. J Neurosci. 1993. 13:4861–4871.50. Bolay H, Dalkara T. Mechanisms of motor dysfunction after transient MCA occlusion: persistent transmission failure in cortical synapses is a major determinant. Stroke. 1998. 29:1988–1993.51. Bolay H, Gürsoy-Ozdemir Y, Sara Y, Onur R, Can A, Dalkara T. Persistent defect in transmitter release and synapsin phosphorylation in cerebral cortex after transient moderate ischemic injury. Stroke. 2002. 33:1369–1375.52. Sun FY, Lin X, Mao LZ, Ge WH, Zhang LM, Huang YL, Gu J. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J Pineal Res. 2002. 33:48–56.53. Hai J, Yu F, Lin Q, Su SH. The changes of signal transduction pathways in hippocampal regions and postsynaptic densities after chronic cerebral hypoperfusion in rats. Brain Res. 2012. 1429:9–17.54. Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007. 27:2846–2857.55. Hu BR, Park M, Martone ME, Fischer WH, Ellisman MH, Zivin JA. Assembly of proteins to postsynaptic densities after transient cerebral ischemia. J Neurosci. 1998. 18:625–633.56. Martone ME, Jones YZ, Young SJ, Ellisman MH, Zivin JA, Hu BR. Modification of postsynaptic densities after transient cerebral ischemia: a quantitative and three-dimensional ultrastructural study. J Neurosci. 1999. 19:1988–1997.57. Meng FJ, Guo J, Song B, Yan XB, Zhang GY. Competitive binding of postsynaptic density 95 and Ca2+-calmodulin dependent protein kinase II to N-methyl-D-aspartate receptor subunit 2B in rat brain. Acta Pharmacol Sin. 2004. 25:176–180.58. Yamada M, Saisu H, Ishizuka T, Takahashi H, Abe T. Immunohistochemical distribution of the two isoforms of synaphin/complexin involved in neurotransmitter release: localization at the distinct central nervous system regions and synaptic types. Neuroscience. 1999. 93:7–18.59. Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SG, Caron MG. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004. 41:625–638.60. Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996. 87:1327–1338.61. Hu BR, Wieloch T. Persistent translocation of Ca2+/calmodulin-dependent protein kinase II to synaptic junctions in the vulnerable hippocampal CA1 region following transient ischemia. J Neurochem. 1995. 64:277–2784.62. Liu Z, Zhao W, Xu T, Pei D, Peng Y. Alterations of NMDA receptor subunits NR1, NR2A and NR2B mRNA expression and their relationship to apoptosis following transient forebrain ischemia. Brain Res. 2010. 1361:133–139.63. Chen WF, Chang H, Wong CS, Huang LT, Yang CH, Yang SN. Impaired expression of postsynaptic density proteins in the hippocampal CA1 region of rats following perinatal hypoxia. Exp Neurol. 2007. 204:400–410.64. Takagi N, Logan R, Teves L, Wallace MC, Gurd JW. Altered interaction between PSD-95 and the NMDA receptor following transient global ischemia. J Neurochem. 2000. 74:169–178.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ERRATUM: Neurons by oxygen-glucose deprivation in organotypic hippocampal slice cultures
- Injury of [correction] Neurons by oxygen-glucose deprivation in organotypic hippocampal slice cultures
- Loss of Parvalbumin Reactive Interneuron in Area CA3 of the Kainate-Treated Mouse Hippocampal Slice Culture
- Calcium Ion Dynamics after Dexamethasone Treatment in Organotypic Cultured Hippocampal Slice
- Estrogen Pretreatment of Organotypic Hippocampal Slices Protects Neurons against Oxygen-Glucose Deprivation with Akt Activation