Effects of Human Mesenchymal Stem Cell Transplantation Combined with Polymer on Functional Recovery Following Spinal Cord Hemisection in Rats
- Affiliations
-
- 1Department of Physiology, Brain Korea 21 Project for Medical Science, and Brain Research Institute, Yonsei University College of Medicine, Seoul 120-752, Korea. bhlee@yuhs.ac
- 2Department of Dental Hygiene, Division of Health Science, Dongseo University, Busan 617-716, Korea.
- 3Department of Anatomy, Ajou University School of Medicine, Suwon 443-721, Korea.
- 4Center for Cell Death Regulating Biodrug, Ajou University School of Medicine, Suwon 443-721, Korea.
- KMID: 2285449
- DOI: http://doi.org/10.4196/kjpp.2012.16.6.405
Abstract
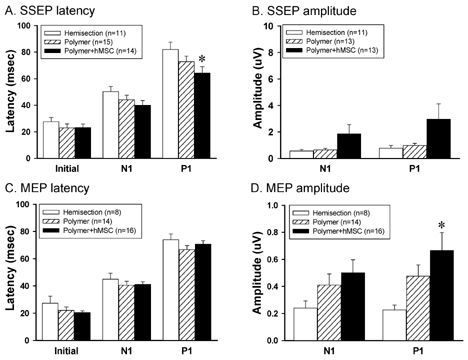
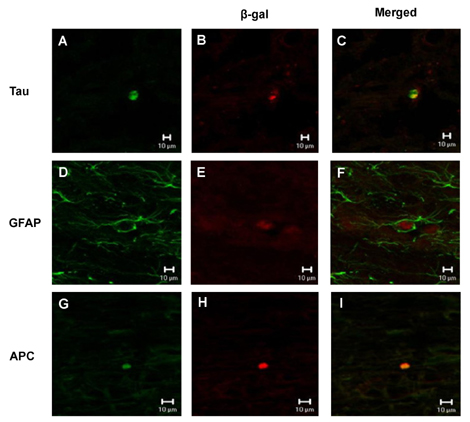
- The spontaneous axon regeneration of damaged neurons is limited after spinal cord injury (SCI). Recently, mesenchymal stem cell (MSC) transplantation was proposed as a potential approach for enhancing nerve regeneration that avoids the ethical issues associated with embryonic stem cell transplantation. As SCI is a complex pathological entity, the treatment of SCI requires a multipronged approach. The purpose of the present study was to investigate the functional recovery and therapeutic potential of human MSCs (hMSCs) and polymer in a spinal cord hemisection injury model. Rats were subjected to hemisection injuries and then divided into three groups. Two groups of rats underwent partial thoracic hemisection injury followed by implantation of either polymer only or polymer with hMSCs. Another hemisection-only group was used as a control. Behavioral, electrophysiological and immunohistochemical studies were performed on all rats. The functional recovery was significantly improved in the polymer with hMSC-transplanted group as compared with control at five weeks after transplantation. The results of electrophysiologic study demonstrated that the latency of somatosensory-evoked potentials (SSEPs) in the polymer with hMSC-transplanted group was significantly shorter than in the hemisection-only control group. In the results of immunohistochemical study, beta-gal-positive cells were observed in the injured and adjacent sites after hMSC transplantation. Surviving hMSCs differentiated into various cell types such as neurons, astrocytes and oligodendrocytes. These data suggest that hMSC transplantation with polymer may play an important role in functional recovery and axonal regeneration after SCI, and may be a potential therapeutic strategy for SCI.
MeSH Terms
Figure
Cited by 3 articles
-
Effect of Phorbol 12-Myristate 13-Acetate on the Differentiation of Adipose-Derived Stromal Cells from Different Subcutaneous Adipose Tissue Depots
Jennifer K. Song, Chang Hoon Lee, So-Min Hwang, Bo Sun Joo, Sun Young Lee, Jin Sup Jung
Korean J Physiol Pharmacol. 2014;18(4):289-296. doi: 10.4196/kjpp.2014.18.4.289.The effect of progesterone and 17-β estradiol on membrane-bound HLA-G in adipose derived stem cells
Akram Moslehi, Batool Hashemi-beni, Azam Moslehi, Maryam Ali Akbari, Minoo Adib
Korean J Physiol Pharmacol. 2016;20(4):341-346. doi: 10.4196/kjpp.2016.20.4.341.Is There Additive Therapeutic Effect When GCSF Combined with Adipose-Derived Stem Cell in a Rat Model of Acute Spinal Cord Injury?
Joongkee Min, Jeong Hoon Kim, Kyoung Hyo Choi, Hyung Ho Yoon, Sang Ryong Jeon
J Korean Neurosurg Soc. 2017;60(4):404-416. doi: 10.3340/jkns.2016.1010.008.
Reference
-
1. Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999. 49:377–391.2. Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996. 76:319–370.3. Liu WG, Wang ZY, Huang ZS. Bone marrow-derived mesenchymal stem cells expressing the bFGF transgene promote axon regeneration and functional recovery after spinal cord injury in rats. Neurol Res. 2011. 33:686–693.4. Schwab ME. Repairing the injured spinal cord. Science. 2002. 295:1029–1031.5. Kang CE, Poon PC, Tator CH, Shoichet MS. A new paradigm for local and sustained release of therapeutic molecules to the injured spinal cord for neuroprotection and tissue repair. Tissue Eng Part A. 2009. 15:595–604.6. Piantino J, Burdick JA, Goldberg D, Langer R, Benowitz LI. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp Neurol. 2006. 201:359–367.7. Houle JD, Ziegler MK. Bridging a complete transection lesion of adult rat spinal cord with growth factor-treated nitrocellulose implants. J Neural Transplant Plast. 1994. 5:115–124.8. Oudega M, Gautier SE, Chapon P, Fragoso M, Bates ML, Parel JM, Bunge MB. Axonal regeneration into Schwann cell grafts within resorbable poly(alpha-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials. 2001. 22:1125–1136.9. Jendelová P, Herynek V, Urdzíková L, Glogarová K, Kroupová J, Andersson B, Bryja V, Burian M, Hájek M, Syková E. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004. 76:232–243.10. Lesný P, De Croos J, Prádný M, Vacík J, Michálek J, Woerly S, Syková E. Polymer hydrogels usable for nervous tissue repair. J Chem Neuroanat. 2002. 23:243–247.11. Woerly S, Doan VD, Evans-Martin F, Paramore CG, Peduzzi JD. Spinal cord reconstruction using NeuroGel implants and functional recovery after chronic injury. J Neurosci Res. 2001. 66:1187–1197.12. Maquet V, Martin D, Scholtes F, Franzen R, Schoenen J, Moonen G, Jér me R. Poly(D,L-lactide) foams modified by poly (ethylene oxide)-block-poly(D,L-lactide) copolymers and a-FGF: in vitro and in vivo evaluation for spinal cord regeneration. Biomaterials. 2001. 22:1137–1146.13. Tsai EC, Dalton PD, Shoichet MS, Tator CH. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transection. J Neurotrauma. 2004. 21:789–804.14. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.15. Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004. 22:377–384.16. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.17. Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001. 56:1666–1672.18. Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001. 18:813–819.19. Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001. 12:559–563.20. Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001. 49:1196–1203.21. Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998. 95:3908–3913.22. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999. 96:10711–10716.23. Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, Lu M, Rosenblum M. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000. 11:3001–3005.24. Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002. 99:2199–2204.25. Yazdani SO, Pedram M, Hafizi M, Kabiri M, Soleimani M, Dehghan MM, Jahanzad I, Gheisari Y, Hashemi SM. A comparison between neurally induced bone marrow derived mesenchymal stem cells and olfactory ensheathing glial cells to repair spinal cord injuries in rat. Tissue Cell. 2012. 44:205–213.26. Zurita M, Vaquero J. Functional recovery in chronic paraplegia after bone marrow stromal cells transplantation. Neuroreport. 2004. 15:1105–1108.27. Ankeny DP, McTigue DM, Jakeman LB. Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp Neurol. 2004. 190:17–31.28. Himes BT, Neuhuber B, Coleman C, Kushner R, Swanger SA, Kopen GC, Wagner J, Shumsky JS, Fischer I. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006. 20:278–296.29. Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromalcells in the injured spinal cord exhibit donor variations. Brain Res. 2005. 1035:73–85.30. Zurita M, Vaquero J. Bone marrow stromal cells can achieve cure of chronic paraplegic rats: functional and morphological outcome one year after transplantation. Neurosci Lett. 2006. 402:51–56.31. Lee KH, Suh-Kim H, Choi JS, Jeun SS, Kim EJ, Kim SS, Yoon do H, Lee BH. Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol Exp (Wars). 2007. 67:13–22.32. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12:1–21.33. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994. 53:55–63.34. Chen G, Hu YR, Wan H, Xia L, Li JH, Yang F, Qu X, Wang SG, Wang ZC. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells and Schwann cells. Chin Med J (Engl). 2010. 123:2424–2431.35. Quertainmont R, Cantinieaux D, Botman O, Sid S, Schoenen J, Franzen R. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS One. 2012. 7:e39500.36. Siddall PJ, Yezierski RP, Loeser JD. Pain following spinal cord injury: clinical features, prevalence, and taxonomy. IASP Newsletter. 2000. 3:3–7.37. Yoon YW, Dong H, Arends JJ, Jacquin MF. Mechanical and cold allodynia in a rat spinal cord contusion model. Somatosens Mot Res. 2004. 21:25–31.38. Sasaki M, Honmou O, Akiyama Y, Uede T, Hashi K, Kocsis JD. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001. 35:26–34.39. Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002. 22:6623–6630.40. Wu S, Suzuki Y, Ejiri Y, Noda T, Bai H, Kitada M, Kataoka K, Ohta M, Chou H, Ide C. Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J Neurosci Res. 2003. 72:343–351.41. Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005. 191:344–360.42. Zhang YQ, He LM, Xing B, Zeng X, Zeng CG, Zhang W, Quan DP, Zeng YS. Neurotrophin-3 gene-modified Schwann cells promote TrkC gene-modified mesenchymal stem cells to differentiate into neuron-like cells in poly(lactic-acid-co-glycolic acid) multiple-channel conduit. Cells Tissues Organs. 2012. 195:313–322.43. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.44. Himes BT, Goldberger ME, Tessler A. Grafts of fetal central nervous system tissue rescue axotomized Clarke's nucleus neurons in adult and neonatal operates. J Comp Neurol. 1994. 339:117–131.45. Himes BT, Liu Y, Solowska JM, Snyder EY, Fischer I, Tessler A. Transplants of cells genetically modified to express neurotrophin-3 rescue axotomized Clarke's nucleus neurons after spinal cord hemisection in adult rats. J Neurosci Res. 2001. 65:549–564.46. Liu Y, Himes BT, Murray M, Tessler A, Fischer I. Grafts of BDNF-producing fibroblasts rescue axotomized rubrospinal neurons and prevent their atrophy. Exp Neurol. 2002. 178:150–164.47. Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002. 1:92–100.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Transplantation of Human Embryonic Stem Cellson Functional Recovery in Spinal Cord Injured Rats
- Olig2-expressing Mesenchymal Stem Cells Enhance Functional Recovery after Contusive Spinal Cord Injury
- The Effect of Human Adipose Tissue Derived Mesenchymal Stem Cells and Growth Hormone on the Recovery of Neurological Deficits due to Experimental Spinal Cord Injury in Rat
- Comparison of the Outcomes after Intralesional, Intracisternal, and Intravenous Transplantation of Human Bone Marrow Derived Mesenchymal Stem Cells for Spinal Cord Injured Rat
- Functional Recovery in Complete Spinal Cord Injury after Transplantation of Human Umbilical Cord Blood Cells in Rats