Korean J Physiol Pharmacol.
2012 Dec;16(6):393-398. 10.4196/kjpp.2012.16.6.393.
Dexamethasone Induces FcgammaRIIb Expression in RBL-2H3 Cells
- Affiliations
-
- 1Research Institute for Medical Sciences and Department of Biochemistry, College of Medicine, Chungnam National University, Daejeon 301-747, Korea. parksk@cnu.ac.kr
- 2Department of Pharmacology, College of Medicine, Chungnam National University, Daejeon 301-747, Korea.
- 3Department of Oriental Medicine, Daejeon University, Daejeon 301-721, Korea.
- 4Division of Life Science, Korea Basic Science Institute, Daejeon 300-716, Korea.
- 5Department of Internal Medicine, Chungnam National University Hospital, Daejeon 305-806, Korea.
- KMID: 2285447
- DOI: http://doi.org/10.4196/kjpp.2012.16.6.393
Abstract
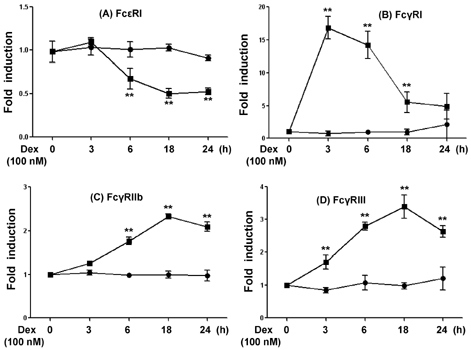
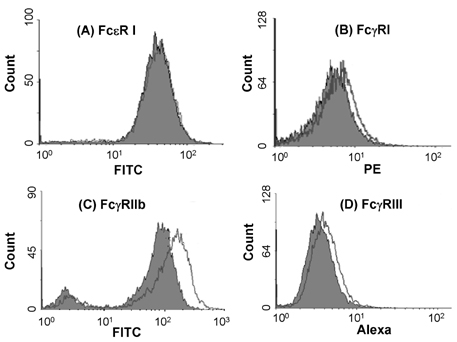
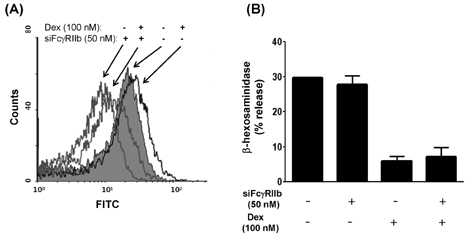
- Mast cells are involved in allergic responses, protection against pathogens and autoimmune diseases. Dexamethasone (Dex) and other glucocorticoids suppress FcepsilonRI-mediated release of inflammatory mediators from mast cells. The inhibition mechanisms were mainly investigated on the downstream signaling of Fc receptor activations. Here, we addressed the effects of Dex on Fc receptor expressions in rat mast cell line RBL-2H3. We measured mRNA levels of Fc receptors by real-time PCR. As expected, Dex decreased the mRNA levels of activating Fc receptor for IgE (FcepsilonR) I and increased the mRNA levels of the inhibitory Fc receptor for IgG FcgammaRIIb. Interestingly, Dex stimulated transcriptions of other activating receptors such as Fc receptors for IgG (FcgammaR) I and FcgammaRIII. To investigate the mechanisms underlying transcriptional regulation, we employed a transcription inhibitor actinomycin D and a translation inhibitor cycloheximide. The inhibition of protein synthesis without Dex treatment enhanced FcgammaRI and FcgammaRIII mRNA levels potently, while FcepsilonRI and FcgammaRIIb were minimally affected. Next, we examined expressions of the Fc receptors on cell surfaces by the flow cytometric method. Only FcgammaRIIb protein expression was significantly enhanced by Dex treatment, while FcgammaRI, FcgammaRIII and FcepsilonRI expression levels were marginally changed. Our data showed, for the first time, that Dex regulates Fc receptor expressions resulting in augmentation of the inhibitory receptor FcgammaRIIb.
Keyword
MeSH Terms
Figure
Reference
-
1. Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000. 191:813–822.2. Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002. 297:1689–1692.3. Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007. 13:719–724.4. Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009. 15:940–945.5. Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002. 2:580–592.6. Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010. 10:328–343.7. Scharenberg AM, Lin S, Cuenod B, Yamamura H, Kinet JP. Reconstitution of interactions between tyrosine kinases and the high affinity IgE receptor which are controlled by receptor clustering. EMBO J. 1995. 14:3385–3394.8. Rosen J, Miner JN. The search for safer glucocorticoid receptor ligands. Endocr Rev. 2005. 26:452–464.9. Clark AR. Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol. 2007. 275:79–97.10. Ito K, Chung KF, Adcock IM. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2006. 117:522–543.11. Smoak K, Cidlowski JA. Glucocorticoids regulate tristetraprolin synthesis and posttranscriptionally regulate tumor necrosis factor alpha inflammatory signaling. Mol Cell Biol. 2006. 26:9126–9135.12. Ing NH. Steroid hormones regulate gene expression posttranscriptionally by altering the stabilities of messenger RNAs. Biol Reprod. 2005. 72:1290–1296.13. Stahn C, Löwenberg M, Hommes DW, Buttgereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007. 275:71–78.14. Stellato C. Post-transcriptional and nongenomic effects of glucocorticoids. Proc Am Thorac Soc. 2004. 1:255–263.15. Hiragun T, Peng Z, Beaven MA. Dexamethasone up-regulates the inhibitory adaptor protein Dok-1 and suppresses downstream activation of the mitogen-activated protein kinase pathway in antigen-stimulated RBL-2H3 mast cells. Mol Pharmacol. 2005. 67:598–603.16. Kassel O, Sancono A, Krätzschmar J, Kreft B, Stassen M, Cato AC. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001. 20:7108–7116.17. Park SK, Beaven MA. Mechanism of upregulation of the inhibitory regulator, src-like adaptor protein (SLAP), by glucocorticoids in mast cells. Mol Immunol. 2009. 46:492–497.18. Hiragun T, Peng Z, Beaven MA. Cutting edge: dexamethasone negatively regulates Syk in mast cells by up-regulating SRC-like adaptor protein. J Immunol. 2006. 177:2047–2050.19. Ozawa K, Szallasi Z, Kazanietz MG, Blumberg PM, Mischak H, Mushinski JF, Beaven MA. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J Biol Chem. 1993. 268:1749–1749.20. Benhamou M, Ninio E, Salem P, Hieblot C, Bessou G, Pitton C, Liu FT, Mencia-Huerta JM. Decrease in IgE Fc receptor expression on mouse bone marrow-derived mast cells and inhibition of paf-acether formation and of beta-hexosaminidase release by dexamethasone. J Immunol. 1986. 136:1385–1392.21. Ovcharenko I, Nobrega MA, Loots GG, Stubbs L. ECR Browser: a tool for visualizing and accessing data from comparisons of multiple vertebrate genomes. Nucleic Acids Res. 2004. 32:W280–W286.22. Yamaguchi M, Hirai K, Komiya A, Miyamasu M, Furumoto Y, Teshima R, Ohta K, Morita Y, Galli SJ, Ra C, Yamamoto K. Regulation of mouse mast cell surface Fc epsilon RI expression by dexamethasone. Int Immunol. 2001. 13:843–851.23. Ishmael FT, Fang X, Galdiero MR, Atasoy U, Rigby WF, Gorospe M, Cheadle C, Stellato C. Role of the RNA-binding protein tristetraprolin in glucocorticoid-mediated gene regulation. J Immunol. 2008. 180:8342–8353.24. Petroni KC, Shen L, Guyre PM. Modulation of human polymorphonuclear leukocyte IgG Fc receptors and Fc receptor-mediated functions by IFN-gamma and glucocorticoids. J Immunol. 1988. 140:3467–3472.25. Pfirsch-Maisonnas S, Aloulou M, Xu T, Claver J, Kanamaru Y, Tiwari M, Launay P, Monteiro RC, Blank U. Inhibitory ITAM signaling traps activating receptors with the phosphatase SHP-1 to form polarized "inhibisome" clusters. Sci Signal. 2011. 4:ra24.26. Kanamaru Y, Pfirsch S, Aloulou M, Vrtovsnik F, Essig M, Loirat C, Deschênes G, Guérin-Marchand C, Blank U, Monteiro RC. Inhibitory ITAM signaling by Fc alpha RI-FcR gamma chain controls multiple activating responses and prevents renal inflammation. J Immunol. 2008. 180:2669–2678.27. Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, Ravetch JV, Takai T. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999. 189:1573–1579.28. Falanga YT, Chaimowitz NS, Charles N, Finkelman FD, Pullen NA, Barbour S, Dholaria K, Faber T, Kolawole M, Huang B, Odom S, Rivera J, Carlyon J, Conrad DH, Spiegel S, Oskeritzian CA, Ryan JJ. Lyn but not Fyn kinase controls IgG-mediated systemic anaphylaxis. J Immunol. 2012. 188:4360–4368.29. Sylvestre DL, Ravetch JV. A dominant role for mast cell Fc receptors in the Arthus reaction. Immunity. 1996. 5:387–390.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Synthetic Chenodeoxycholic Acid Derivative, HS-1200-induced Apoptosis of RBL-2H3 Cells
- Fluoxetine-induced Changes on Activity of Tryptophan Hydroxylase at RBL-2H3 Cells
- Intracellular Ca2+ Mobilization and Beta-hexosaminidase Release Are Not Influenced by 60 Hz-electromagnetic Fields (EMF) in RBL 2H3 Cells
- Inhibition of Interleukin-4 and β-Hexosaminidase Release in RBL-2H3 Cells by Compounds Isolated from Lobelia chinensis
- Anti-Oxidative and Anti-Inflammatory Effect of Water Extract from Perillae semen in RBL-2H3 Cells