Korean J Physiol Pharmacol.
2011 Dec;15(6):437-443. 10.4196/kjpp.2011.15.6.437.
c-Fos Expression in the Nucleus of the Solitary Tract in Response to Salt Stimulation in Rats
- Affiliations
-
- 1Department of Physiology, Yonsei University College of Medicine, Seoul 120-752, Korea. bhlee@yuhs.ac
- 2Department of Anesthesiology and Pain Medicine, Yonsei University College of Medicine, Seoul 120-752, Korea.
- 3BK 21 Project for Medical Science, Yonsei University College of Medicine, Seoul 120-752, Korea.
- 4Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul 120-752, Korea.
- 5Functional Food Technology Research Group, Korea Food Research Institute, Sungnam 463-746, Korea.
- KMID: 2285432
- DOI: http://doi.org/10.4196/kjpp.2011.15.6.437
Abstract
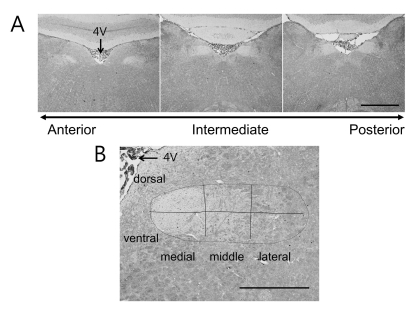
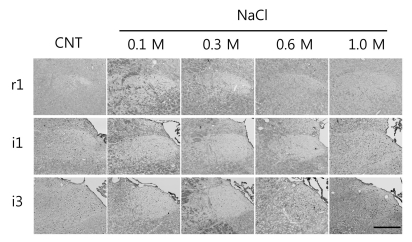
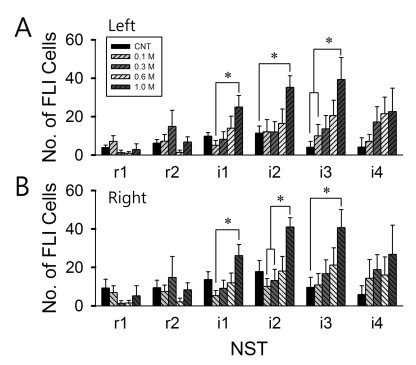
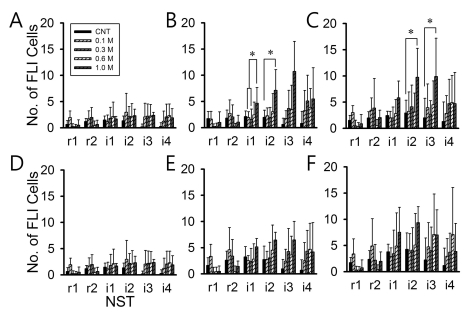
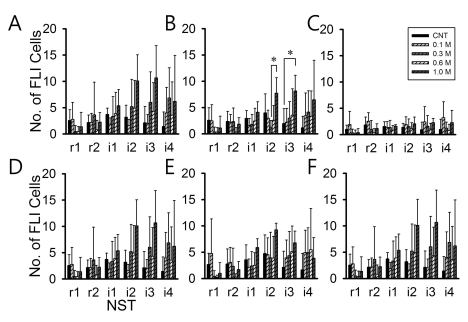
- Salt signals in tongue are relayed to the nucleus of the solitary tract (NST). This signaling is very important to determine whether to swallow salt-related nutrition or not and suggests some implications in discrimination of salt concentration. Salt concentration-dependent electrical responses in the chorda tympani and the NST were well reported. But salt concentration-dependency and spatial distribution of c-Fos in the NST were not well established. In the present study, NaCl signaling in the NST was studied in urethane-anesthetized rats. The c-Fos immunoreactivity in the six different NST areas along the rostral-caudal axis and six subregions in each of bilateral NST were compared between applications of distilled water and different concentrations of NaCl to the tongue of experimental animals. From this study, salt stimulation with high concentration (1.0 M NaCl) induced significantly higher c-Fos expression in intermediate NST and dorsal-medial and dorsal-middle subregions of the NST compared to distilled water stimulation. The result represents the specific spatial distribution of salt taste perception in the NST.
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Acupuncture Stimulation at Different Acupoints on Formalin-Induced Pain in Rats
Kyung Ha Chang, Sun Joon Bai, Hyejung Lee, Bae Hwan Lee
Korean J Physiol Pharmacol. 2014;18(2):121-127. doi: 10.4196/kjpp.2014.18.2.121.
Reference
-
1. Paxinos G. The Rat Nervous System. 2004. 3rd ed. San Diego: Elsevier Academic Press.2. Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984; 222:560–577. PMID: 6199385.
Article3. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006; 443:289–295. PMID: 16988703.
Article4. Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996; 50:83–107. PMID: 8971979.5. Chen JY, Victor JD, Di Lorenzo PM. Temporal coding of intensity of NaCl and HCl in the nucleus of the solitary tract of the rat. J Neurophysiol. 2011; 105:697–711. PMID: 21106899.
Article6. Carstens E, Sudo S, Jinks S, Simons C, Dressier JM, Carstens MI. Activation of neurons in trigeminal subnucleus caudalis (Vc) by irritant chemical stimulation. Methods in Chemosensory Research. 2002. CRC Press.
Article7. Chan CY, Yoo JE, Travers SP. Diverse bitter stimuli elicit highly similar patterns of Fos-like immunoreactivity in the nucleus of the solitary tract. Chem Senses. 2004; 29:573–581. PMID: 15337683.
Article8. Yamamoto T, Sawa K. c-Fos-like immunoreactivity in the brainstem following gastric loads of various chemical solutions in rats. Brain Res. 2000; 866:135–143. PMID: 10825489.
Article9. Harrer MI, Travers SP. Topographic organization of Fos-like immunoreactivity in the rostral nucleus of the solitary tract evoked by gustatory stimulation with sucrose and quinine. Brain Res. 1996; 711:125–137. PMID: 8680855.
Article10. Travers SP. Quinine and citric acid elicit distinctive Fos-like immunoreactivity in the rat nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2002; 282:R1798–R1810. PMID: 12010763.11. Contreras RJ. Cagan RH, editor. Gustatory mechanisms of a specific appetite. Neural mechanisms of taste. 1989. Boca Raton, FL: CRC Press;p. 119–145.
Article12. Lindemann B. Receptors and transduction in taste. Nature. 2001; 413:219–225. PMID: 11557991.
Article13. Duncan CJ. Salt preferences of birds and mammals. Physiol Zoology. 1962; 35:120–132.
Article14. Schafe GE, Fitts DA, Thiele TE, LeDoux JE, Bernstein IL. The induction of c-Fos in the NTS after taste aversion learning is not correlated with measures of conditioned fear. Behav Neurosci. 2000; 114:99–106. PMID: 10718265.
Article15. Houpt TA, Smith GP, Joh TH, Frankmann SP. c-fos-like immunoreactivity in the subfornical organ and nucleus of the solitary tract following salt intake by sodium-depleted rats. Physiol Behav. 1998; 63:505–510. PMID: 9523891.
Article16. Grancha ML, Navarro M, Cubero I, Thiele TE, Bernstein IL. Induction of a brainstem correlate of conditioned taste aversion expression: role of the pontine parabrachial nucleus. Behav Brain Res. 2002; 131:205–209. PMID: 11844587.
Article17. Travers SP, Hu H. Extranuclear projections of rNST neurons expressing gustatory-elicited Fos. J Comp Neurol. 2000; 427:124–138. PMID: 11042595.
Article18. Stratford JM, Finger TE. Central representation of postingestive chemosensory cues in mice that lack the ability to taste. J Neurosci. 2011; 31:9101–9110. PMID: 21697361.
Article19. King CT, Garcea M, Spector AC. Glossopharyngeal nerve regeneration is essential for the complete recovery of quinine-stimulated oromotor rejection behaviors and central patterns of neuronal activity in the nucleus of the solitary tract in the rat. J Neurosci. 2000; 20:8426–8434. PMID: 11069950.
Article20. Scott TR, Giza BK. Coding channels in the taste system of the rat. Science. 1990; 249:1585–1587. PMID: 2171145.
Article21. Corson SL, Hill DL. Chorda tympani nerve terminal field maturation and maintenance is severely altered following changes to gustatory nerve input to the nucleus of the solitary tract. J Neurosci. 2011; 31:7591–7603. PMID: 21613473.
Article22. Takayama K, Suzuki T, Miura M. The comparison of effects of various anesthetics on expression of Fos protein in the rat brain. Neurosci Lett. 1994; 176:59–62. PMID: 7970238.
Article23. Rosen AM, Roussin AT, Di Lorenzo PM. Water as an independent taste modality. Front Neurosci. 2010; 4:175. PMID: 21048894.
Article24. Voorhies AC, Bernstein IL. Induction and expression of salt appetite: effects on Fos expression in nucleus accumbens. Behav Brain Res. 2006; 172:90–96. PMID: 16712968.
Article25. Navarro M, Spray KJ, Cubero I, Thiele TE, Bernstein IL. cFos induction during conditioned taste aversion expression varies with aversion strength. Brain Res. 2000; 887:450–453. PMID: 11134640.
Article26. Houpt TA, Philopena JM, Wessel TC, Joh TH, Smith GP. Increased c-fos expression in nucleus of the solitary tract correlated with conditioned taste aversion to sucrose in rats. Neurosci Lett. 1994; 172:1–5. PMID: 8084508.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- c-Fos-like Immunoreactivity in the nucleus of the solitary tract following taste stimulation of the contralateral side of the tongue
- Fos Protein Expression in Trigeminal Nociceptive Central Pathway of the Rat Brain by Cisternal Capsaicin Injection
- The Role of the Vestibular System in Modulating Blood Pressure of Sinoaortic Denervated Rats
- Fos Expression Induced by Combined Injection of Leptin and Cholecystokinin in the Rat Brain
- Nociceptive and Trigeminal Cardiovascular Reflex Mechanisms after Noxious Intrapulpal Stimulation of the Rat Incisors