Korean J Physiol Pharmacol.
2011 Dec;15(6):431-436. 10.4196/kjpp.2011.15.6.431.
Alteration of Ryanodine-receptors in Cultured Rat Aortic Smooth Muscle Cells
- Affiliations
-
- 1Department of Physiology, College of Medicine, Konyang University, Daejeon 302-718, Korea. sehkim@konyang.ac.kr, hspark@konyang.ac.kr
- 2Myunggok Medical Research Institute, Konyang University, Daejeon 302-718, Korea.
- KMID: 2285431
- DOI: http://doi.org/10.4196/kjpp.2011.15.6.431
Abstract
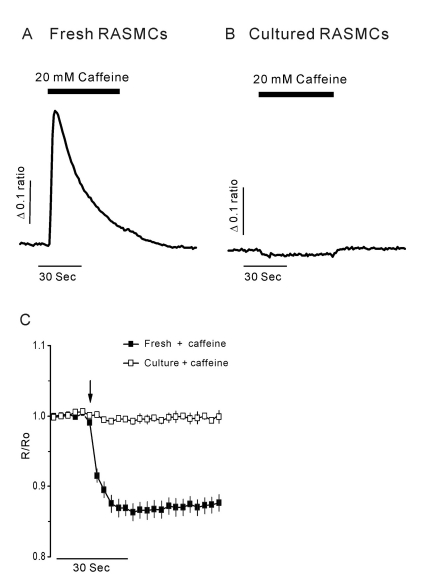
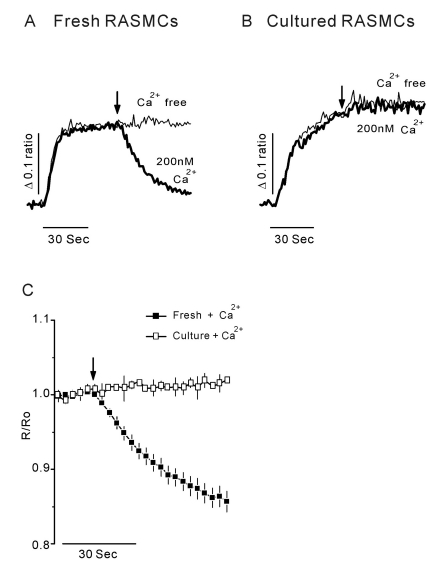
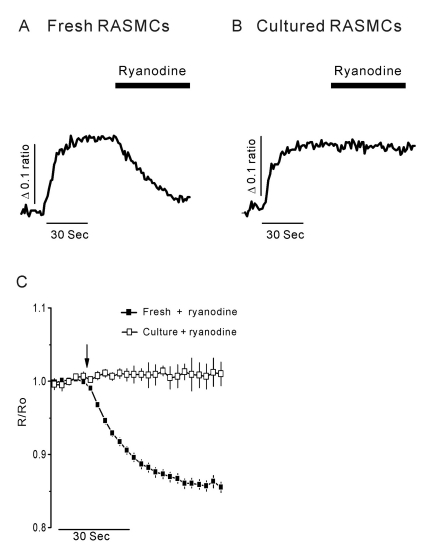
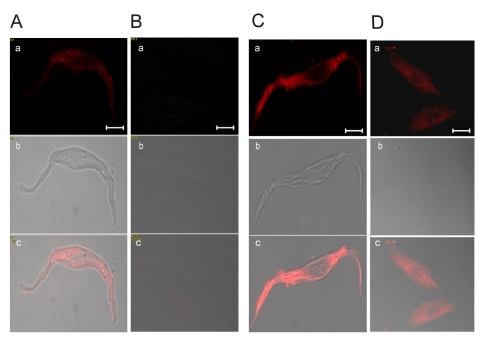
- Vascular smooth muscle cells can obtain a proliferative function in environments such as atherosclerosis in vivo or primary culture in vitro. Proliferation of vascular smooth muscle cells is accompanied by changes in ryanodine receptors (RyRs). In several studies, the cytosolic Ca2+ response to caffeine is decreased during smooth muscle cell culture. Although caffeine is commonly used to investigate RyR function because it is difficult to measure Ca2+ release from the sarcoplasmic reticulum (SR) directly, caffeine has additional off-target effects, including blocking inositol trisphosphate receptors and store-operated Ca2+ entry. Using freshly dissociated rat aortic smooth muscle cells (RASMCs) and cultured RASMCs, we sought to provide direct evidence for the operation of RyRs through the Ca2+- induced Ca2+-release pathway by directly measuring Ca2+ release from SR in permeabilized cells. An additional goal was to elucidate alterations of RyRs that occurred during culture. Perfusion of permeabilized, freshly dissociated RASMCs with Ca2+ stimulated Ca2+ release from the SR. Caffeine and ryanodine also induced Ca2+ release from the SR in dissociated RASMCs. In contrast, ryanodine, caffeine and Ca2+ failed to trigger Ca2+ release in cultured RASMCs. These results are consistent with results obtained by immunocytochemistry, which showed that RyRs were expressed in dissociated RASMCs, but not in cultured RASMCs. This study is the first to demonstrate Ca2+ release from the SR by cytosolic Ca2+ elevation in vascular smooth muscle cells, and also supports previous studies on the alterations of RyRs in vascular smooth muscle cells associated with culture.
MeSH Terms
-
Animals
Atherosclerosis
Caffeine
Cell Culture Techniques
Cytosol
Immunohistochemistry
Inositol
Muscle, Smooth
Muscle, Smooth, Vascular
Myocytes, Smooth Muscle
Perfusion
Rats
Ryanodine
Ryanodine Receptor Calcium Release Channel
Sarcoplasmic Reticulum
Caffeine
Inositol
Ryanodine
Ryanodine Receptor Calcium Release Channel
Figure
Reference
-
1. Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979; 59:1–61. PMID: 108688.
Article2. Schwartz SM, Campbell GR, Campbell JH. Replication of smooth muscle cells in vascular disease. Circ Res. 1986; 58:427–444. PMID: 3516443.
Article3. Halayko AJ, Salari H, MA X, Stephens NL. Markers of airway smooth muscle cell phenotype. Am J Physiol. 1996; 270:L1040–L1051. PMID: 8764231.
Article4. Thyberg J. Differentiated properties and proliferation of arterial smooth muscle cells in culture. Int Rev Cytol. 1996; 169:183–265. PMID: 8843655.
Article5. Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993; 361:315–325. PMID: 8381210.
Article6. Ghosh TK, Bian JH, Short AD, Rybak SL, Gill DL. Persistent intracellular calcium pool depletion by thapsigargin and its influence on cell growth. J Biol Chem. 1991; 266:24690–24697. PMID: 1761564.
Article7. Short AD, Bian J, Ghosh TK, Waldron RT, Rybak SL, Gill DL. Intracellular Ca2+ pool content is linked to control of cell growth. Proc Natl Acad Sci USA. 1993; 90:4986–4990. PMID: 8389460.8. Waldron RT, Short AD, Meadows JJ, Ghosh TK, Gill DL. Endoplasmic reticulum calcium pump expression and control of cell growth. J Biol Chem. 1994; 269:11927–11933. PMID: 8163492.
Article9. Wang Y, Chen J, Wang Y, Taylor CW, Hirata Y, Hagiwara H, Mikoshiba K, Toyo-oka T, Omata M, Sakaki Y. Crucial role of type 1, but not type 3, inositol 1,4,5-trisphosphate (IP3) receptors in IP3-induced Ca2+ release, capacitative Ca2+ entry, and proliferation of A7r5 vascular smooth muscle cells. Circ Res. 2001; 88:202–209. PMID: 11157673.10. Savineau JP. Is the translocon a crucial player of the calcium homeostasis in vascular smooth muscle cell? Am J Physiol Heart Circ Physiol. 2009; 296:H906–H907. PMID: 19252099.
Article11. Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J. 2007; 30:114–133. PMID: 17601970.12. Côrtes SF, Lemos VS, Stoclet JC. Alterations in calcium stores in aortic myocytes from spontaneously hypertensive rats. Hypertension. 1997; 29:1322–1328. PMID: 9180636.
Article13. Côrtes SF, Lemos VS, Corriu C, Stoclet JC. Changes in angiotensin II receptor density and calcium handling during proliferation in SHR aortic myocytes. Am J Physiol. 1996; 271:H2330–H2338. PMID: 8997290.14. Choi KJ, Kim KS, Kim SH, Kim DK, Park HS. Caffeine and 2-aminoethoxydiphenyl borate (2-APB) have different ability to inhibit intracellular calcium mobilization in pancreatic acinar cell. Korean J Physiol Pharmacol. 2010; 14:105–111. PMID: 20473382.
Article15. Hume JR, McAllister CE, Wilson SM. Caffeine inhibits InsP3 responses and capacitative calcium entry in canine pulmonary arterial smooth muscle cells. Vascul Pharmacol. 2009; 50:89–97. PMID: 19084078.16. Islam MS. The ryanodine receptor calcium channel of beta-cells: molecular regulation and physiological significance. Diabetes. 2002; 51:1299–1309. PMID: 11978625.17. Kamishima T, Quayle JM. Ca2+-induced Ca2+ release in cardiac and smooth muscle cells. Biochem Soc Trans. 2003; 31:943–946. PMID: 14505454.18. Choi KJ, Cho DS, Kim JY, Kim BJ, Lee KM, Kim SH, Kim DK, Kim SH, Park HS. Ca2+-induced Ca2+ release from internal stores in INS-1 rat insulinoma cells. Korean J Physiol Pharmacol. 2011; 15:53–59. PMID: 21461241.19. Masumiya H, Li P, Zhang L, Chen SR. Ryanodine sensitizes the Ca2+ release channel (ryanodine receptor) to CaCa2+ activation. J Biol Chem. 2001; 276:39727–39735. PMID: 11507100.20. Vallot O, Combettes L, Jourdon P, Inamo J, Marty I, Claret M, Lompré AM. Intracellular Ca2+ handling in vascular smooth muscle cells is affected by proliferation. Arterioscler Thromb Vasc Biol. 2000; 20:1225–1235. PMID: 10807737.21. Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008; 295:C779–C790. PMID: 18596214.22. Perez CF, Voss A, Pessah IN, Allen PD. RyR1/RyR3 chimeras reveal that multiple domains of RyR1 are involved in skeletal-type E-C coupling. Biophys J. 2003; 84:2655–2663. PMID: 12668474.
Article23. Copello JA, Barg S, Sonnleitner A, Porta M, Diaz-Sylvester P, Fill M, Schindler H, Fleischer S. Differential activation by Ca2+, ATP and caffeine of cardiac and skeletal muscle ryanodine receptors after block by Mg2+. J Membr Biol. 2002; 187:51–64. PMID: 12029377.24. Fessenden JD, Wang Y, Moore RA, Chen SR, Allen PD, Pessah IN. Divergent functional properties of ryanodine receptor types 1 and 3 expressed in a myogenic cell line. Biophys J. 2000; 79:2509–2525. PMID: 11053126.
Article25. Nakai J, Ogura T, Protasi F, Franzini-Armstrong C, Allen PD, Beam KG. Functional nonequality of the cardiac and skeletal ryanodine receptors. Proc Natl Acad Sci USA. 1997; 94:1019–1022. PMID: 9023375.
Article26. Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995; 270:633–637. PMID: 7570021.
Article27. Dreja K, Nordström I, Hellstrand P. Rat arterial smooth muscle devoid of ryanodine receptor function: effects on cellular Ca2+ handling. Br J Pharmacol. 2001; 132:1957–1966. PMID: 11309269.28. Arnould T, Vankoningsloo S, Renard P, Houbion A, Ninane N, Demazy C, Remacle J, Raes M. CREB activation induced by mitochondrial dysfunction is a new signaling pathway that impairs cell proliferation. EMBO J. 2002; 21:53–63. PMID: 11782425.
Article29. Giebler HA, Lemasson I, Nyborg JK. p53 recruitment of CREB binding protein mediated through phosphorylated CREB: a novel pathway of tumor suppressor regulation. Mol Cell Biol. 2000; 20:4849–4858. PMID: 10848610.
Article30. Wilkerson MK, Heppner TJ, Bonev AD, Nelson MT. Inositol trisphosphate receptor calcium release is required for cerebral artery smooth muscle cell proliferation. Am J Physiol Heart Circ Physiol. 2006; 290:H240–H247. PMID: 16113072.
Article31. Kusner LL, Mygland A, Kaminski HJ. Ryanodine receptor gene expression thymomas. Muscle Nerve. 1998; 21:1299–1303. PMID: 9736058.
Article32. Jaggar JH, Nelson MT. Differential regulation of Ca2+ sparks and Ca2+ waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2000; 279:C1528–C1539. PMID: 11029300.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of sodium vanadate on contractility of vascular smooth muscle
- The effect of anti-hypertensive drugs on DNA synthesis and proliferation of ultured rat aortic smooth muscle cells
- Proliferation of Cultured Vascular Smooth Muscle Cells(VSMCs) Obtained from Aortas of Insulin Dependent Diabetic Rats
- Proliferative Ability of Aortic Smooth Muscle Cells and Lipid Peroxidation of Red Blood Cell Membrane in Diabetic Rats
- In Vitro Culture of Endothelial Cell and Smooth Muscle Cell for Studying Vascular Diseases