Korean J Physiol Pharmacol.
2011 Dec;15(6):345-351. 10.4196/kjpp.2011.15.6.345.
4-phenylbutyric Acid Regulates Collagen Synthesis and Secretion Induced by High Concentrations of Glucose in Human Gingival Fibroblasts
- Affiliations
-
- 1Department of Dental Pharmacology and Wonkwang Dental Research Institute, School of Dentistry, Wonkwang University, Iksan 570-711, Korea. hrkimdp@wonkwang.ac.kr
- 2Department of Public Oral Health and Preventive Dentistry, School of Dentistry, Wonkwang University, Iksan 570-711, Korea.
- 3Department of Oral Medicine and Diagnosis, School of Dentistry, Wonkwang University, Iksan 570-711, Korea.
- 4Department of Oral and Maxillofacial Radiology, School of Dentistry, Wonkwang University, Iksan 570-711, Korea.
- 5Department of Pharmacology and Institute of Cardiovascular Research, Medical School, Chonbuk National University, Jeonju 561-181, Korea. hjchae@chonbuk.ac.kr
- KMID: 2285421
- DOI: http://doi.org/10.4196/kjpp.2011.15.6.345
Abstract
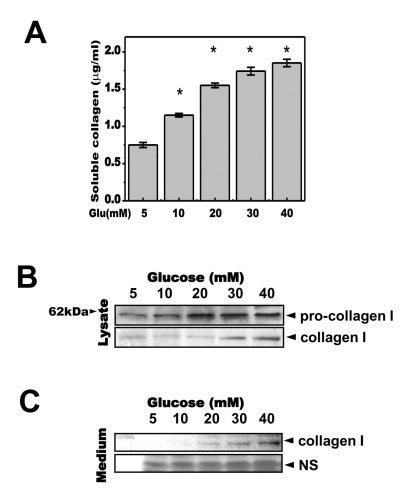
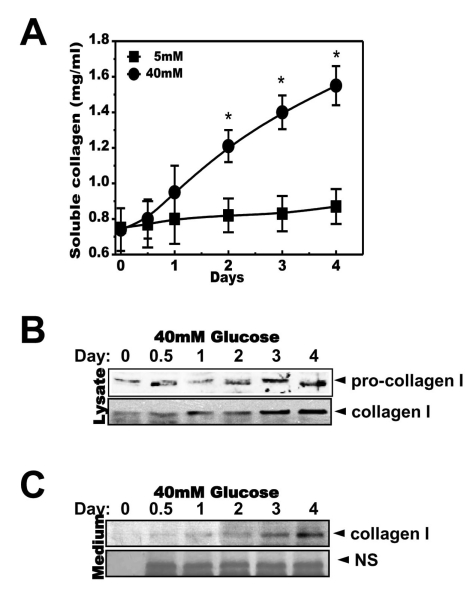
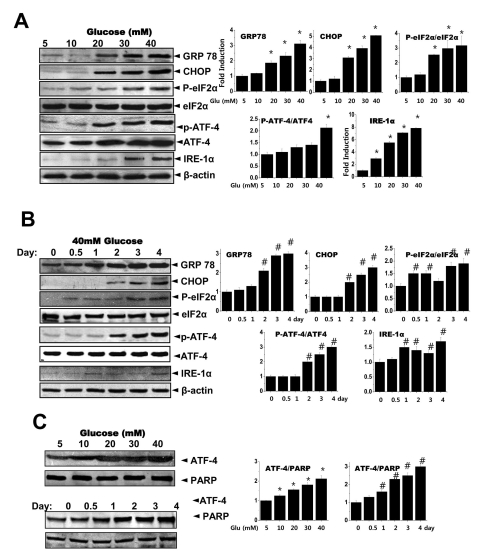
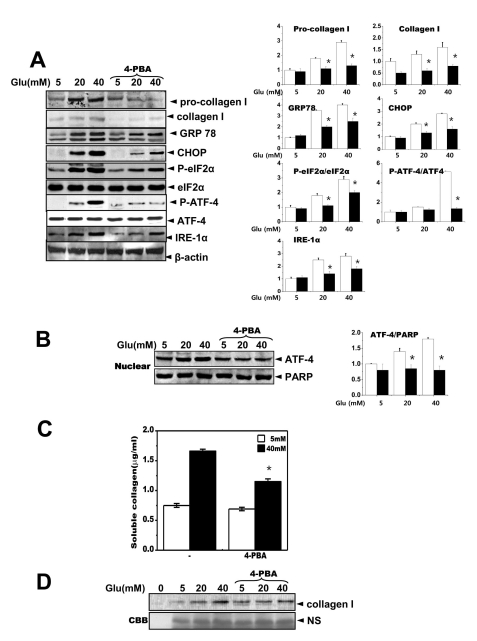
- High glucose leads to physio/pathological alterations in diabetes patients. We investigated collagen production in human gingival cells that were cultured in high concentrations of glucose. Collagen synthesis and secretion were increased when the cells were exposed to high concentrations of glucose. We examined endoplasmic reticulum (ER) stress response because glucose metabolism is related to ER functional status. An ER stress response including the expression of glucose regulated protein 78 (GRP78), C/EBP homologous protein (CHOP), inositol requiring enzyme alpha (IRE-1alpha) and phosphoreukaryotic initiation factor alpha (p-eIF-2alpha) was activated in the presence of high glucose. Activating transcription factor 4 (ATF-4), a downstream protein of p-eIF-2alpha as well as a transcription factor for collagen, was also phosphorylated and translocalized into the nucleus. The chemical chaperone 4-PBA inhibited the ER stress response and ATF-4 phosphorylation as well as nuclear translocation. Our results suggest that high concentrations of glucose-induced collagen are linked to ER stress and the associated phosphorylation and nuclear translocation of ATF-4.
Keyword
MeSH Terms
-
Activating Transcription Factor 4
Butylamines
Collagen
Endoplasmic Reticulum
Fibroblasts
Glucose
Humans
Inositol
Peptide Initiation Factors
Phenylbutyrates
Phosphorylation
Transcription Factors
Activating Transcription Factor 4
Butylamines
Collagen
Glucose
Inositol
Peptide Initiation Factors
Phenylbutyrates
Transcription Factors
Figure
Cited by 1 articles
-
Effect of Exercise Intensity on Unfolded Protein Response in Skeletal Muscle of Rat
Kihoon Kim, Yun-Hye Kim, Sung-Hye Lee, Man-Joong Jeon, So-Young Park, Kyung-Oh Doh
Korean J Physiol Pharmacol. 2014;18(3):211-216. doi: 10.4196/kjpp.2014.18.3.211.
Reference
-
1. Melloul D, Marshak S, Cerasi E. Regulation of insulin gene transcription. Diabetologia. 2002; 45:309–326. PMID: 11914736.
Article2. Poitout V, Hagman D, Stein R, Artner I, Robertson RP, Harmon JS. Regulation of the insulin gene by glucose and fatty acids. J Nutr. 2006; 136:873–876. PMID: 16549443.
Article3. Seo HY, Kim YD, Lee KM, Min AK, Kim MK, Kim HS, Won KC, Park JY, Lee KU, Choi HS, Park KG, Lee IK. Endoplasmic reticulum stress-induced activation of activating transcription factor 6 decreases insulin gene expression via up-regulation of orphan nuclear receptor small heterodimer partner. Endocrinology. 2008; 149:3832–3841. PMID: 18450959.
Article4. Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006; 4:245–254. PMID: 16950141.
Article5. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529. PMID: 17565364.
Article6. Zhang L, Lai E, Teodoro T, Volchuk A. GRP78, but not protein-disulfide isomerase, partially reverses hyperglycemia-induced inhibition of insulin synthesis and secretion in pancreatic {beta}-cells. J Biol Chem. 2009; 284:5289–5298. PMID: 19103594.7. Lee HB, Ha H. Experimental approaches to diabetic nephropathy. Kidney Int. 1997; 52(Suppl 60):S1–S2.8. Perfumo F, Altieri P, Degl'Innocenti ML, Ghiggeri GM, Caridi G, Trivelli A, Gusmano R. Effects of peritoneal effluents on mesothelial cells in culture: cell proliferation and extracellular matrix regulation. Nephrol Dial Transplant. 1996; 11:1803–1809. PMID: 8918626.9. Kumano K, Schiller B, Hjelle JT, Moran J. Effects of osmotic solutes on fibronectin mRNA expression in rat peritoneal mesothelial cells. Blood Purif. 1996; 14:165–169. PMID: 8785032.
Article10. Silva JA, Lorencini M, Reis JR, Carvalho HF, Cagnon VH, Stach-Machado DR. The influence of type I diabetes mellitus in periodontal disease induced changes of the gingival epithelium and connective tissue. Tissue Cell. 2008; 40:283–292. PMID: 18439638.
Article11. Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. 2001; 6:99–112. PMID: 11887478.
Article12. Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996; 1:109–115. PMID: 9222596.
Article13. Kajimura K, Takagi Y, Ueba N, Yamasaki K, Sakagami Y, Yokoyama H, Yoneda K. Protective effect of Astragali Radix by intraperitoneal injection against Japanese encephalitis virus infection in mice. Biol Pharm Bull. 1996; 19:855–859. PMID: 8799486.
Article14. Zhao JH, Liu HL, Lin HY, Huang CH, Fang HW, Chen SS, Ho Y, Tsai WB, Chen WY. Chemical chaperone and inhibitor discovery: potential treatments for protein conformational diseases. Perspect Medicin Chem. 2007; 1:39–48. PMID: 19812735.
Article15. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006; 313:1137–1140. PMID: 16931765.
Article16. Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J Clin Invest. 1997; 100:2457–2465. PMID: 9366560.
Article17. Tanaka T, Tomaru Y, Nomura Y, Miura H, Suzuki M, Hayashizaki Y. Comprehensive search for HNF-1beta-regulated genes in mouse hepatoma cells perturbed by transcription regulatory factor-targeted RNAi. Nucleic Acids Res. 2004; 32:2740–2750. PMID: 15148361.18. Rieusset J. Mitochondria and endoplasmic reticulum: mitochondria-endoplasmic reticulum interplay in type 2 diabetes pathophysiology. Int J Biochem Cell Biol. 2011; 43:1257–1262. PMID: 21605696.
Article19. Roque T, Boncoeur E, Saint-Criq V, Bonvin E, Clement A, Tabary O, Jacquot J. Proinflammatory effect of sodium 4-phenylbutyrate in deltaF508-cystic fibrosis transmembrane conductance regulator lung epithelial cells: involvement of extracellular signal-regulated protein kinase 1/2 and c-Jun-NH2-terminal kinase signaling. J Pharmacol Exp Ther. 2008; 326:949–956. PMID: 18574003.20. Adler S. Structure-function relationships associated with extracellular matrix alterations in diabetic glomerulopathy. J Am Soc Nephrol. 1994; 5:1165–1172. PMID: 7873725.
Article21. Lee GH, Yan C, Shin SJ, Hong SC, Ahn T, Moon A, Park SJ, Lee YC, Yoo WH, Kim HT, Kim DS, Chae SW, Kim HR, Chae HJ. BAX inhibitor-1 enhances cancer metastasis by altering glucose metabolism and activating the sodium-hydrogen exchanger: the alteration of mitochondrial function. Oncogene. 2010; 29:2130–2141. PMID: 20118983.
Article22. Kim ES, Kim JS, Kim SG, Hwang S, Lee CH, Moon A. Sphingosine 1-phosphate regulates matrix metalloproteinase-9 expression and breast cell invasion through S1P3-Gαq coupling. J Cell Sci. 2011; 124:2220–2230. PMID: 21652634.
Article23. Del Prete D, Anglani F, Forino M, Ceol M, Fioretto P, Nosadini R, Baggio B, Gambaro G. Down-regulation of glomerular matrix metalloproteinase-2 gene in human NIDDM. Diabetologia. 1997; 40:1449–1454. PMID: 9447953.
Article24. McLennan SV, Kelly DJ, Cox AJ, Cao Z, Lyons JG, Yue DK, Gilbert RE. Decreased matrix degradation in diabetic nephropathy: effects of ACE inhibition on the expression and activities of matrix metalloproteinases. Diabetologia. 2002; 45:268–275. PMID: 11935159.
Article25. Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999; 397:271–274. PMID: 9930704.
Article26. Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998; 18:7499–7509. PMID: 9819435.27. Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000; 5:897–904. PMID: 10882126.
Article28. Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA. 2004; 101:11269–11274. PMID: 15277680.
Article29. Yoshizawa T, Hinoi E, Jung DY, Kajimura D, Ferron M, Seo J, Graff JM, Kim JK, Karsenty G. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009; 119:2807–2817. PMID: 19726872.
Article30. Disthabanchong S, Radinahamed P, Stitchantrakul W, Hongeng S, Rajatanavin R. Chronic metabolic acidosis alters osteoblast differentiation from human mesenchymal stem cells. Kidney Int. 2007; 71:201–209. PMID: 17183249.
Article31. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006; 313:1137–1140. PMID: 16931765.
Article32. Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol. 2004; 66:899–908. PMID: 15226415.
Article33. Rubenstein RC, Zeitlin PL. Sodium 4-phenylbutyrate downregulates Hsc70: implications for intracellular trafficking of DeltaF508-CFTR. Am J Physiol Cell Physiol. 2000; 278:C259–C267. PMID: 10666020.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Glucose and Insulin on Human Gingival Fibroblasts and Periodontal Ligament Cells
- Effect of Estrogen on Fibroblast Proliferation and Collagen Synthesis
- The Effect of Glucose Control on DNA and Collagen Synthesis of Cultured Fibroblasts of Chronic Diabetic Wounds
- Effect of Granulocyte-macrophage Colony-stimulating Factor and Ascorbic Acid Co-supplementation on the Fibroblast Proliferation and Collagen Synthesis
- The Effects of Ascorbic Acids on the Proliferation of the Human & Rabbit Tenon's Fibroblasts