Korean J Physiol Pharmacol.
2011 Apr;15(2):107-114. 10.4196/kjpp.2011.15.2.107.
Suppression of Autophagy and Activation of Glycogen Synthase Kinase 3beta Facilitate the Aggregate Formation of Tau
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Kangwon National University, Chunchon 200-701, Korea. wchun@kangwon.ac.kr
- 2Department of Anesthesiology, University of Rochester, Rochester, NY, 14642, USA.
- KMID: 2285415
- DOI: http://doi.org/10.4196/kjpp.2011.15.2.107
Abstract
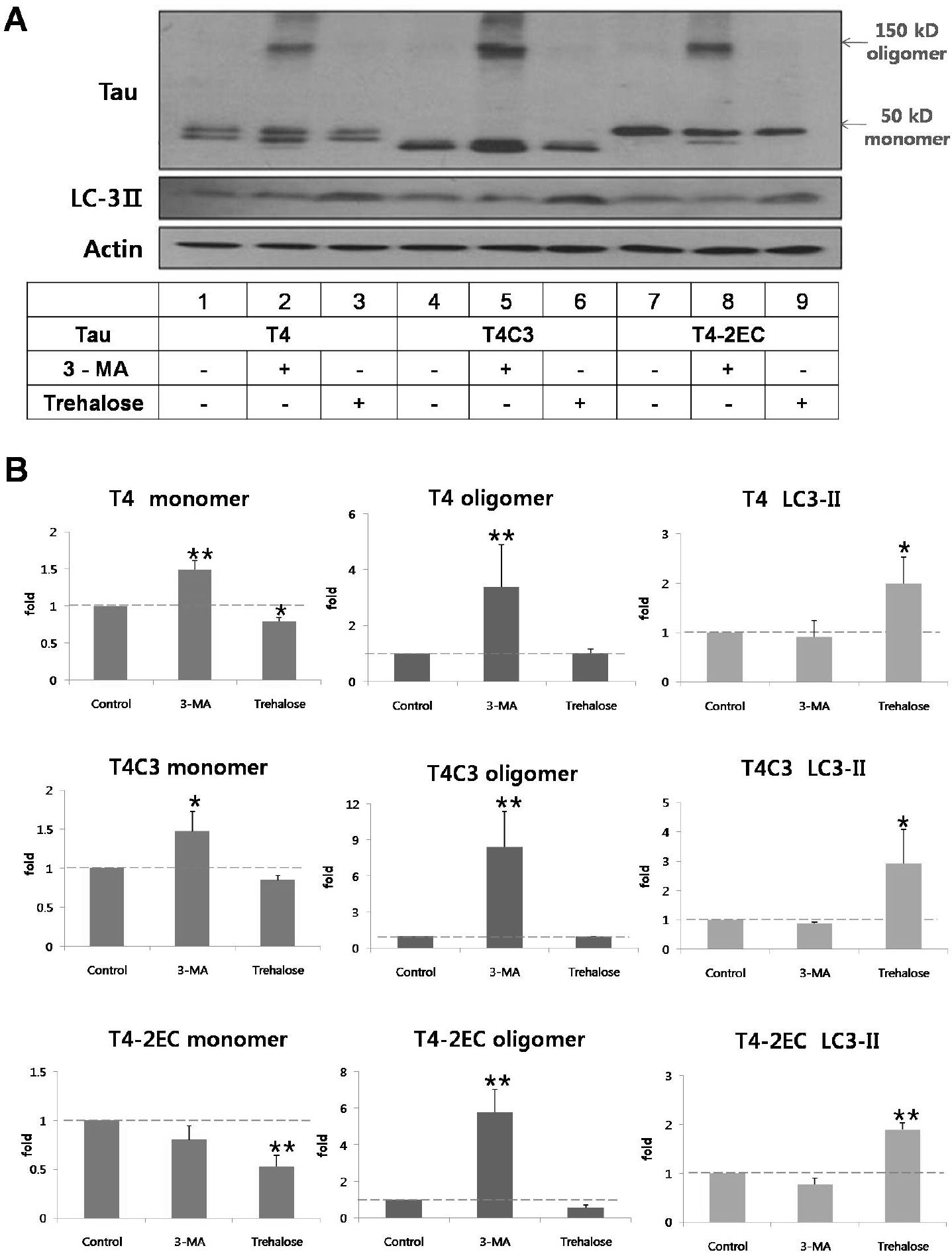
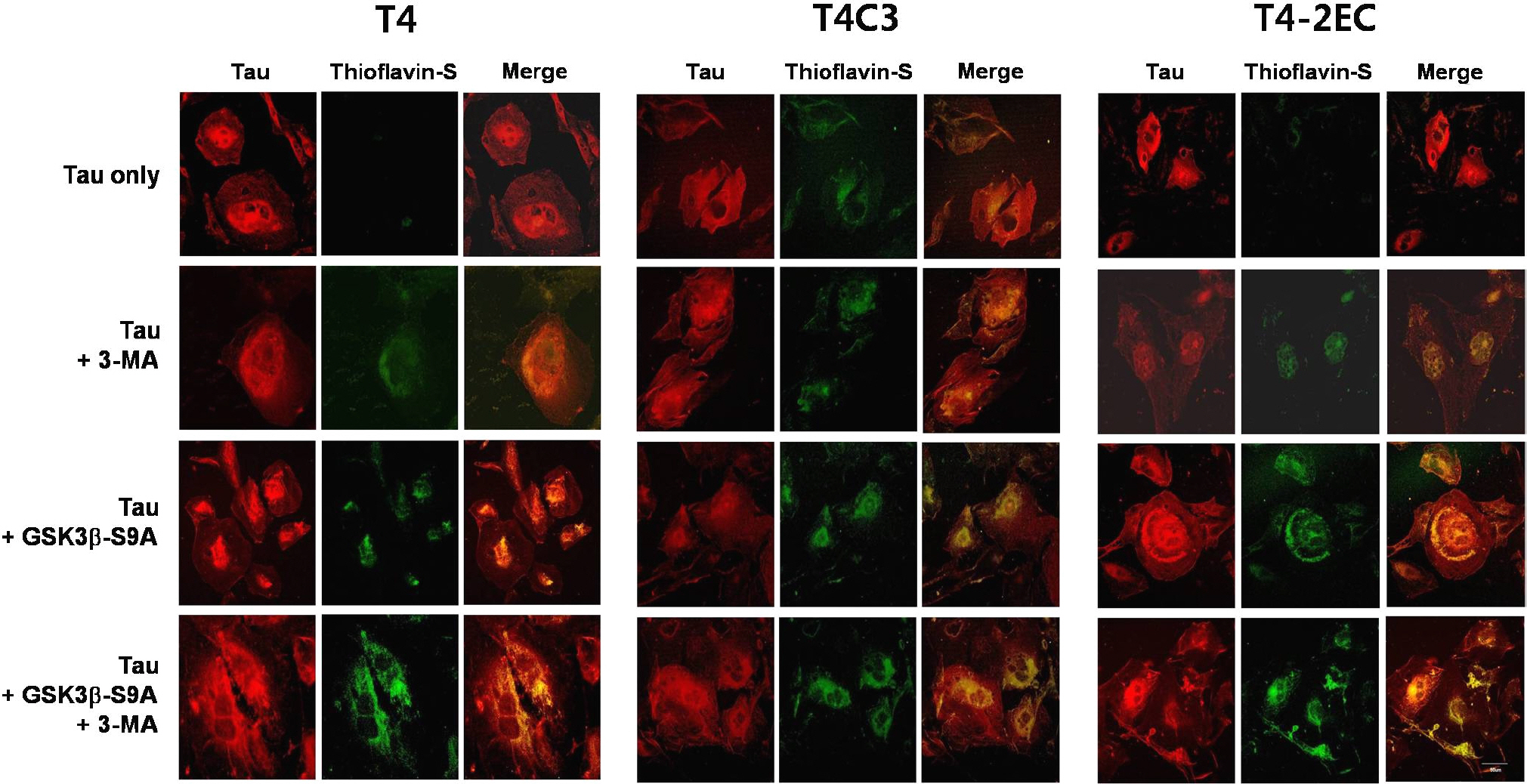
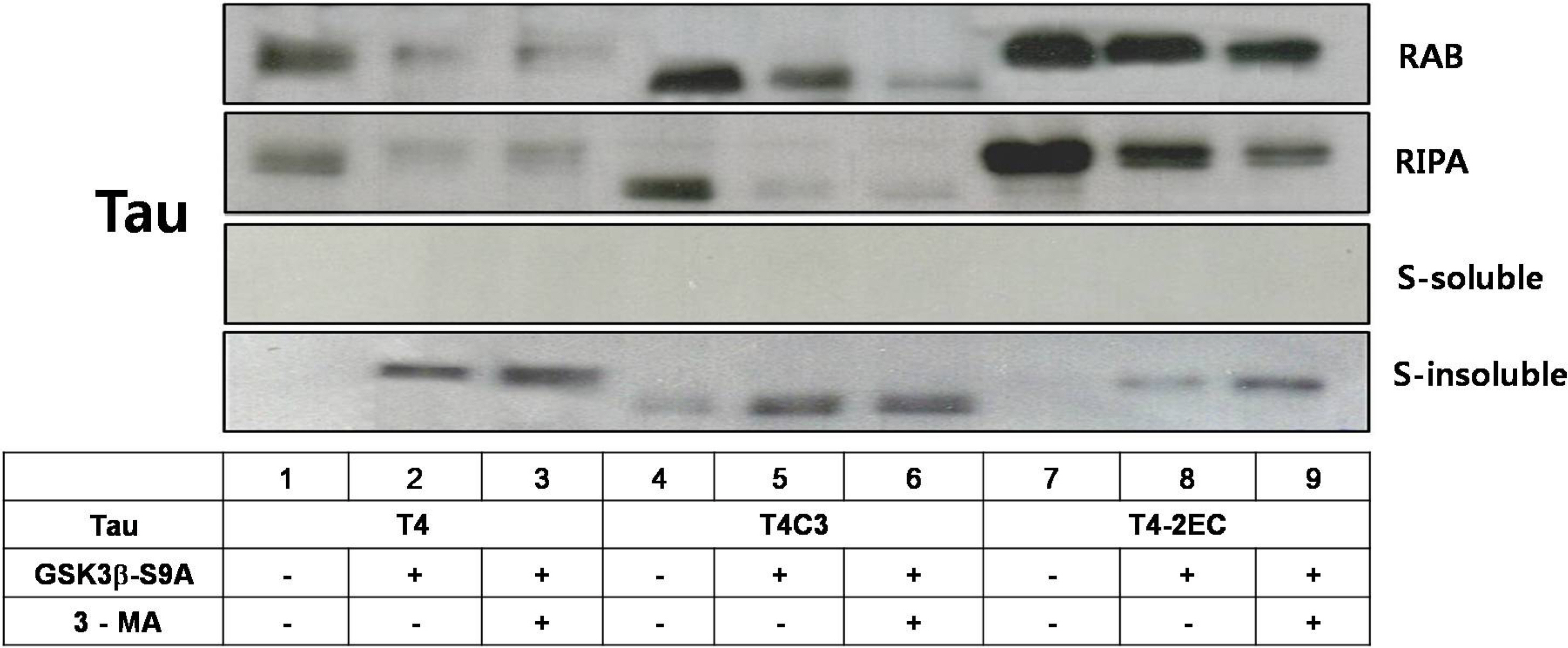
- Neurofibrillary tangle (NFT) is a characteristic hallmark of Alzheimer's disease. GSK3beta has been reported to play a major role in the NFT formation of tau. Dysfunction of autophagy might facilitate the aggregate formation of tau. The present study examined the role of GSK3beta-mediated phosphorylation of tau species on their autophagic degradation. We transfected wild type tau (T4), caspase-3-cleaved tau at Asp421 (T4C3), or pseudophosphorylated tau at Ser396/Ser404 (T4-2EC) in the presence of active or enzyme-inactive GSK3beta. Trehalose and 3-methyladenine (3-MA) were used to enhance or inhibit autophagic activity, respectively. All tau species showed increased accumulation with 3-MA treatment whereas reduced with trehalose, indicating that tau undergoes autophagic degradation. However, T4C3 and T4-2EC showed abundant formation of oligomers than T4. Active GSK3beta in the presence of 3-MA resulted in significantly increased formation of insoluble tau aggregates. These results indicate that GSK3beta-mediated phosphorylation and compromised autophagic activity significantly contribute to tau aggregation.
MeSH Terms
Figure
Cited by 2 articles
-
Sequestration of sorcin by aberrant forms of tau results in the defective calcium homeostasis
Song-In Kim, Hee Jae Lee, Sung-Soo Kim, Yong-Soo Kwon, Wanjoo Chun
Korean J Physiol Pharmacol. 2016;20(4):387-397. doi: 10.4196/kjpp.2016.20.4.387.Novel functional roles of caspase-related genes in the regulation of apoptosis and autophagy
Ju-Hyun Shin, Sang-Hyun Min
Korean J Physiol Pharmacol. 2016;20(6):573-580. doi: 10.4196/kjpp.2016.20.6.573.
Reference
-
References
1. Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986; 83:4913–4917.
Article2. Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004; 29:95–102.
Article3. Lovestone S, Hartley CL, Pearce J, Anderton BH. Phosphorylation of tau by glycogen synthase kinase-3 beta in intact mammalian cells: the effects on the organization and stability of microtubules. Neuroscience. 1996; 73:1145–1157.4. Leroy K, Boutajangout A, Authelet M, Woodgett JR, Anderton BH, Brion JP. The active form of glycogen synthase kinase-3beta is associated with granulovacuolar degeneration in neurons in Alzheimer's disease. Acta Neuropathol. 2002; 103:91–99.5. Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci USA. 2003; 100:10032–10037.
Article6. Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW. Caspasecleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004; 114:121–130.
Article7. Lee HG, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Nunomura A, Smith MA. Tau phosphorylation in Alzheimer's disease: pathogen or protector? Trends Mol Med. 2005; 11:164–169.
Article8. Lee YJ, Kim NY, Suh YA, Lee C. Involvement of ROS in curcumin-induced autophagic cell death. Korean J Physiol Pharmacol. 2010; 15:1–7.
Article9. Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006; 443:780–786.
Article10. Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochim Biophys Acta. 2009; 1792:3–13.
Article11. Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006; 441:880–884.
Article12. Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, Nixon RA. Properties of the endosomal-lysosomal system in the human central nervous system: disturbances mark most neurons in populations at risk to degenerate in Alzheimer's disease. J Neurosci. 1996; 16:186–199.
Article13. Nakamura Y, Takeda M, Suzuki H, Hattori H, Tada K, Hariguchi S, Hashimoto S, Nishimura T. Abnormal distribution of cathepsins in the brain of patients with Alzheimer's disease. Neurosci Lett. 1991; 130:195–198.
Article14. Ding H, Matthews TA, Johnson GV. Site-specific phosphorylation and caspase cleavage differentially impact tau-microtubule interactions and tau aggregation. J Biol Chem. 2006; 281:19107–19114.
Article15. Chun W, Johnson GV. Activation of glycogen synthase kinase 3beta promotes the intermolecular association of tau. The use of fluorescence resonance energy transfer microscopy. J Biol Chem. 2007; 282:23410–23417.16. Cho JH, Johnson GV. Glycogen synthase kinase 3 beta induces caspase-cleaved tau aggregation in situ. J Biol Chem. 2004; 279:54716–54723.17. Sun A, Nguyen XV, Bing G. Comparative analysis of an improved thioflavin-s stain, Gallyas silver stain, and immunohistochemistry for neurofibrillary tangle demonstration on the same sections. J Histochem Cytochem. 2002; 50:463–472.
Article18. Wang Y, Martinez-Vicente M, Krüger U, Kaushik S, Wong E, Mandelkow EM, Cuervo AM, Mandelkow E. Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum Mol Genet. 2009; 18:4153–4170.
Article19. Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alphasynuclein. J Biol Chem. 2007; 282:5641–5652.20. Chun W, Waldo GS, Johnson GV. Split GFP complementation assay: a novel approach to quantitatively measure aggregation of tau in situ: effects of GSK3beta activation and caspase 3 cleavage. J Neurochem. 2007; 103:2529–2539.21. Sato S, Tatebayashi Y, Akagi T, Chui DH, Murayama M, Miyasaka T, Planel E, Tanemura K, Sun X, Hashikawa T, Yoshioka K, Ishiguro K, Takashima A. Aberrant tau phosphorylation by glycogen synthase kinase-3beta and JNK3 induces oligomeric tau fibrils in COS-7 cells. J Biol Chem. 2002; 277:42060–42065.22. Kuret J, Chirita CN, Congdon EE, Kannanayakal T, Li G, Necula M, Yin H, Zhong Q. Pathways of tau fibrillization. Biochim Biophys Acta. 2005; 1739:167–178.
Article23. Feuillette S, Blard O, Lecourtois M, Frebourg T, Campion D, Dumanchin C. Tau is not normally degraded by the proteasome. J Neurosci Res. 2005; 80:400–405.
Article24. McCray BA, Taylor JP. The role of autophagy in age-related neurodegeneration. Neurosignals. 2008; 16:75–84.
Article25. Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, Ko LW. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci. 2008; 27:1119–1130.
Article26. Rankin CA, Sun Q, Gamblin TC. Pseudo-phosphorylation of tau at Ser202 and Thr205 affects tau filament formation. Brain Res Mol Brain Res. 2005; 138:84–93.
Article27. Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry. 1999; 38:3549–3558.
Article28. Poppek D, Keck S, Ermak G, Jung T, Stolzing A, Ullrich O, Davies KJ, Grune T. Phosphorylation inhibits turnover of the tau protein by the proteasome: influence of RCAN1 and oxidative stress. Biochem J. 2006; 400:511–520.
Article29. Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001; 98:6923–6928.30. Chun W, Johnson GV. The role of tau phosphorylation and cleavage in neuronal cell death. Front Biosci. 2007; 12:733–756.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Glycogen Synthase Kinase-3beta mRNA Level in the Hippocampus of Patients with Major Depression: A Study Using the Stanley Neuropathology Consortium Integrative Database
- Immunohistochemical Study on the Distribution of Glycogen Synthase Kinase (GSK) 3beta in the Central Nervous System of SOD1G93A Transgenic Mice
- Phosphorylation of glycogen synthase kinase-3beta at serine-9 by phospholipase Cgamma1 through protein kinase C in rat 3Y1 fibroblasts
- Regulatory B Subunits of Protein Phosphatase 2A Are Involved in Site-specific Regulation of Tau Protein Phosphorylation
- PS-341-Induced Apoptosis is Related to JNK-Dependent Caspase 3 Activation and It is Negatively Regulated by PI3K/Akt-Mediated Inactivation of Glycogen Synthase Kinase-3beta in Lung Cancer Cells