Korean J Physiol Pharmacol.
2011 Apr;15(2):95-100. 10.4196/kjpp.2011.15.2.95.
Regulation of DREAM Expression by Group I mGluR
- Affiliations
-
- 1Department of Pharmacology, Yonsei University College of Medicine, Seoul 120-752, Korea. kimhoon@yuhs.ac
- 2Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul 120-752, Korea.
- 3Division of Metabolic Disease, Department of Biomedical Science, National Institutes of Health, Osong 363-951, Korea.
- 4Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul 120-752, Korea.
- KMID: 2285413
- DOI: http://doi.org/10.4196/kjpp.2011.15.2.95
Abstract
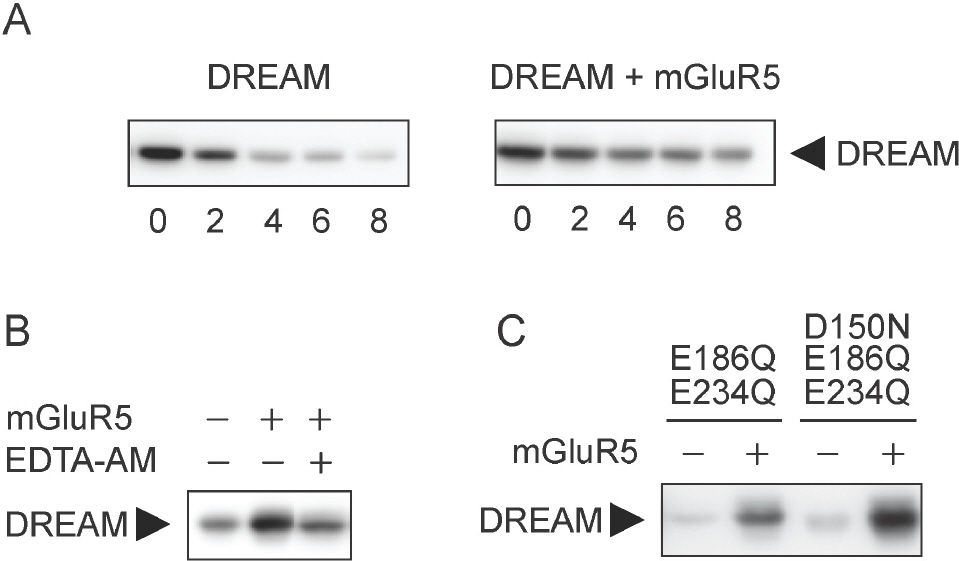
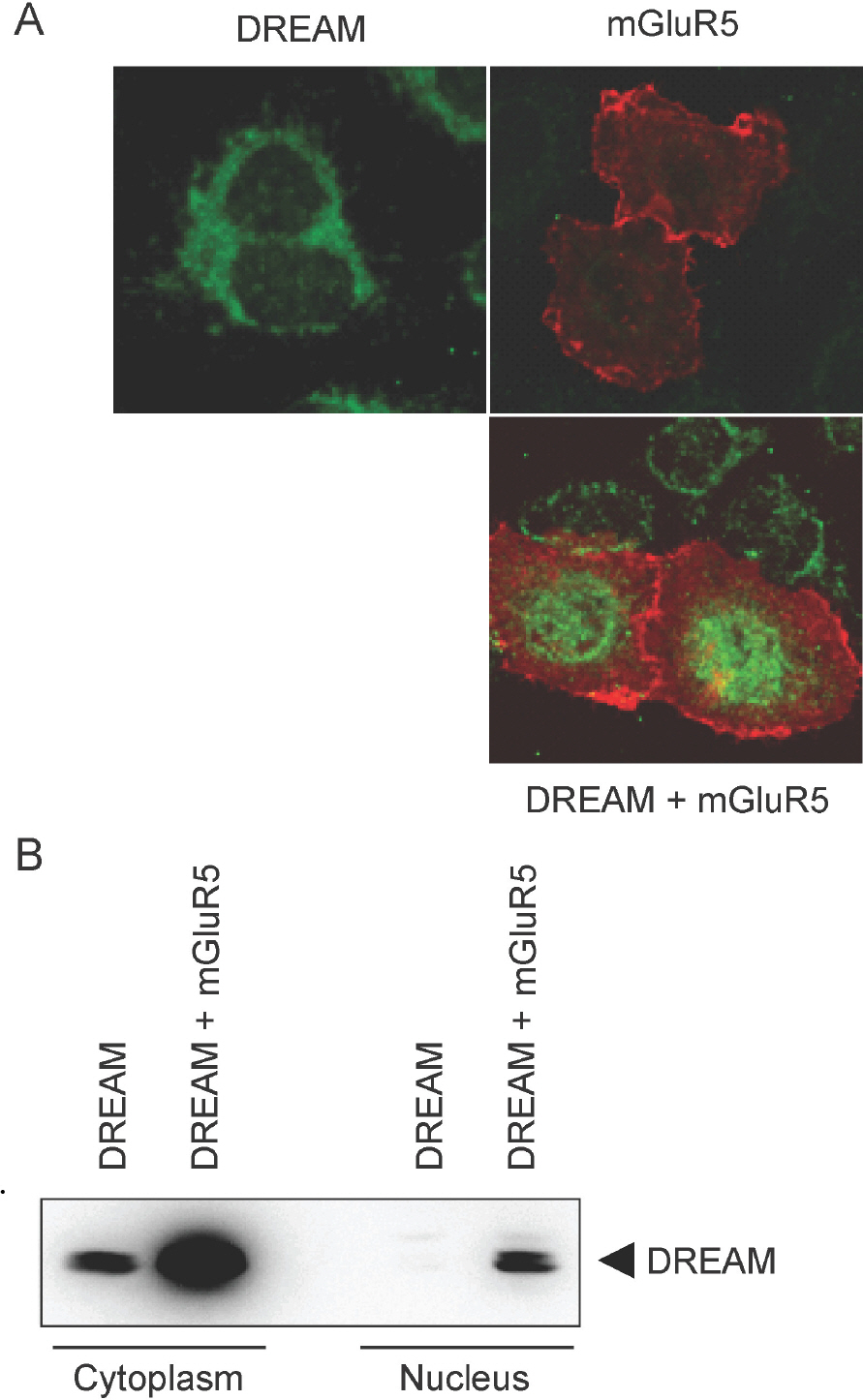
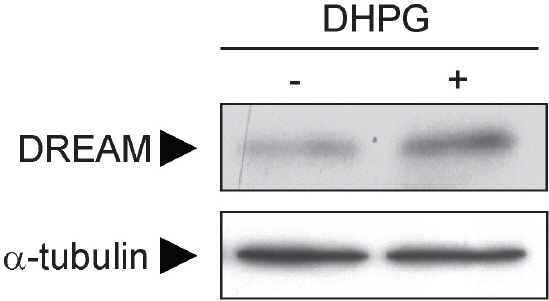
- DREAM (downstream regulatory element antagonistic modulator) is a calcium-binding protein that regulates dynorphin expression, promotes potassium channel surface expression, and enhances presenilin processing in an expression level-dependent manner. However, no molecular mechanism has yet explained how protein levels of DREAM are regulated. Here we identified group I mGluR (mGluR1/5) as a positive regulator of DREAM protein expression. Overexpression of mGluR1/5 increased the cellular level of DREAM. Up-regulation of DREAM resulted in increased DREAM protein in both the nucleus and cytoplasm, where the protein acts as a transcriptional repressor and a modulator of its interacting proteins, respectively. DHPG (3,5-dihydroxyphenylglycine), a group I mGluR agonist, also up-regulated DREAM expression in cortical neurons. These results suggest that group I mGluR is the first identified receptor that may regulate DREAM activity in neurons.
MeSH Terms
Figure
Cited by 1 articles
-
The Effects of Pre-emptive Administration of Ketamine and norBNI on Pain Behavior, c-Fos, and Prodynorphin Protein Expression in the Rat Spinal Cord after Formalin-induced Pain Is Modulated by the DREAM Protein
Idris Long, Rapeah Suppian, Zalina Ismail
Korean J Pain. 2013;26(3):255-264. doi: 10.3344/kjp.2013.26.3.255.
Reference
-
References
1. Buxbaum JD. A role for calsenilin and related proteins in multiple aspects of neuronal function. Biochem Biophys Res Commun. 2004; 322:1140–1144.
Article2. Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, Sarosi I, Woodgett JR, Oliveira-dos-Santos AJ, Ikura M, van der Kooy D, Salter MW, Penninger JM. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002; 108:31–43.
Article3. Buxbaum JD, Choi EK, Luo Y, Lilliehook C, Crowley AC, Merriam DE, Wasco W. Calsenilin: a calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment. Nat Med. 1998; 4:1177–1181.
Article4. An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000; 403:553–556.
Article5. Carrión AM, Link WA, Ledo F, Mellström B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999; 398:80–84.6. Jo DG, Lee JY, Hong YM, Song S, Mook-Jung I, Koh JY, Jung YK. Induction of pro-apoptotic calsenilin/DREAM/KChIP3 in Alzheimer's disease and cultured neurons after amyloid-beta exposure. J Neurochem. 2004; 88:604–611.7. Zaidi NF, Thomson EE, Choi EK, Buxbaum JD, Wasco W. Intracellular calcium modulates the nuclear translocation of calsenilin. J Neurochem. 2004; 89:593–601.
Article8. 8. Woo HN, Chang JW, Choi YH, Gwon AR, Jung YK, Jo DG. Characterization of subcellular localization and Ca2+ modulation of calsenilin/DREAM/KChIP3. Neuroreport. 2008; 19:1193–1197.9. Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010; 50:295–322.
Article10. Ribeiro FM, Paquet M, Cregan SP, Ferguson SS. Group I metabotropic glutamate receptor signalling and its implication in neurological disease. CNS Neurol Disord Drug Targets. 2010; 9:574–595.11. Dölen G, Carpenter RL, Ocain TD, Bear MF. Mechanism-based approaches to treating fragile X. Pharmacol Ther. 2010; 127:78–93.
Article12. Cho CH, Shin HK. Spinal metabotropic glutamate receptors (mGluRs) are involved in the melittin-induced nociception in rats. Korean J Physiol Pharmacol. 2008; 12:237–243.
Article13. Krystal JH, Mathew SJ, D'Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2010; 24:669–693.
Article14. Brackmann M, Zhao C, Kuhl D, Manahan-Vaughan D, Braunewell KH. MGluRs regulate the expression of neuronal calcium sensor proteins NCS-1 and VILIP-1 and the immediate early gene arg3.1/arc in the hippocampus in vivo. Biochem Biophys Res Commun. 2004; 322:1073–1079.15. Osawa M, Dace A, Tong KI, Valiveti A, Ikura M, Ames JB. Mg2+ and Ca2+ differentially regulate DNA binding and dimerization of DREAM. J Biol Chem. 2005; 280:18008–18014.16. Francesconi A, Duvoisin RM. Role of the second and third intracellular loops of metabotropic glutamate receptors in mediating dual signal transduction activation. J Biol Chem. 1998; 273:5615–5624.
Article17. Lee JH, Lee J, Choi KY, Hepp R, Lee JY, Lim MK, Chatani-Hinze M, Roche PA, Kim DG, Ahn YS, Kim CH, Roche KW. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proc Natl Acad Sci USA. 2008; 105:12575–12580.
Article18. Burgoyne RD. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci. 2007; 8:182–193.19. McGarrigle D, Huang XY. GPCRs signaling directly through Src-family kinases. Sci STKE. 2007; 2007::p. e35.
Article20. Sun Y, McGarrigle D, Huang XY. When a G protein-coupled receptor does not couple to a G protein. Mol Biosyst. 2007; 3:849–854.
Article21. Costigan M, Woolf CJ. No DREAM, No pain. Closing the spinal gate. Cell. 2002; 108:297–300.22. Kolber BJ, Montana MC, Carrasquillo Y, Xu J, Heinemann SF, Muglia LJ, Gereau RW 4th. Activation of metabotropic glutamate receptor 5 in the amygdala modulates pain-like behavior. J Neurosci. 2010; 30:8203–8213.
Article23. Walker K, Bowes M, Panesar M, Davis A, Gentry C, Kesingland A, Gasparini F, Spooren W, Stoehr N, Pagano A, Flor PJ, Vranesic I, Lingenhoehl K, Johnson EC, Varney M, Urban L, Kuhn R. Metabotropic glutamate receptor subtype 5 (mGlu5) and nociceptive function. I. Selective blockade of mGlu5 receptors in models of acute, persistent and chronic pain. Neuropharmacology. 2001; 40:1–9.24. Sevostianova N, Danysz W. Analgesic effects of mGlu1 and mGlu5 receptor antagonists in the rat formalin test. Neuropharmacology. 2006; 51:623–630.
Article25. Hu HJ, Alter BJ, Carrasquillo Y, Qiu CS, Gereau RW 4th. Metabotropic glutamate receptor 5 modulates nociceptive plasticity via extracellular signal-regulated kinase-Kv4.2 signaling in spinal cord dorsal horn neurons. J Neurosci. 2007; 27:13181–13191.