Korean J Physiol Pharmacol.
2010 Jun;14(3):169-176. 10.4196/kjpp.2010.14.3.169.
Hyperosmotic Stimulus Down-regulates 1alpha, 25-dihydroxyvitamin D3-induced Osteoclastogenesis by Suppressing the RANKL Expression in a Co-culture System
- Affiliations
-
- 1Department of Oral Biology, Yonsei University, Seoul 120-752, Korea. lsi@yuhs.ac
- 2Department of Orthodontics, Yonsei University, Seoul 120-752, Korea.
- 3Oral Science Research Center, Yonsei University, Seoul 120-752, Korea.
- 4Brain Korea 21 Project of Dental Science, Yonsei University, Seoul 120-752, Korea.
- 5College of Dentistry, Yonsei University, Seoul 120-752, Korea.
- 6Department of Oral Microbiology, College of Dentistry, Chonnam National University, Gwangju 500-757, Korea.
- 7Department of Physiology, College of Medicine, Chungnam National University, Daejeon 305-764, Korea.
- KMID: 2285399
- DOI: http://doi.org/10.4196/kjpp.2010.14.3.169
Abstract
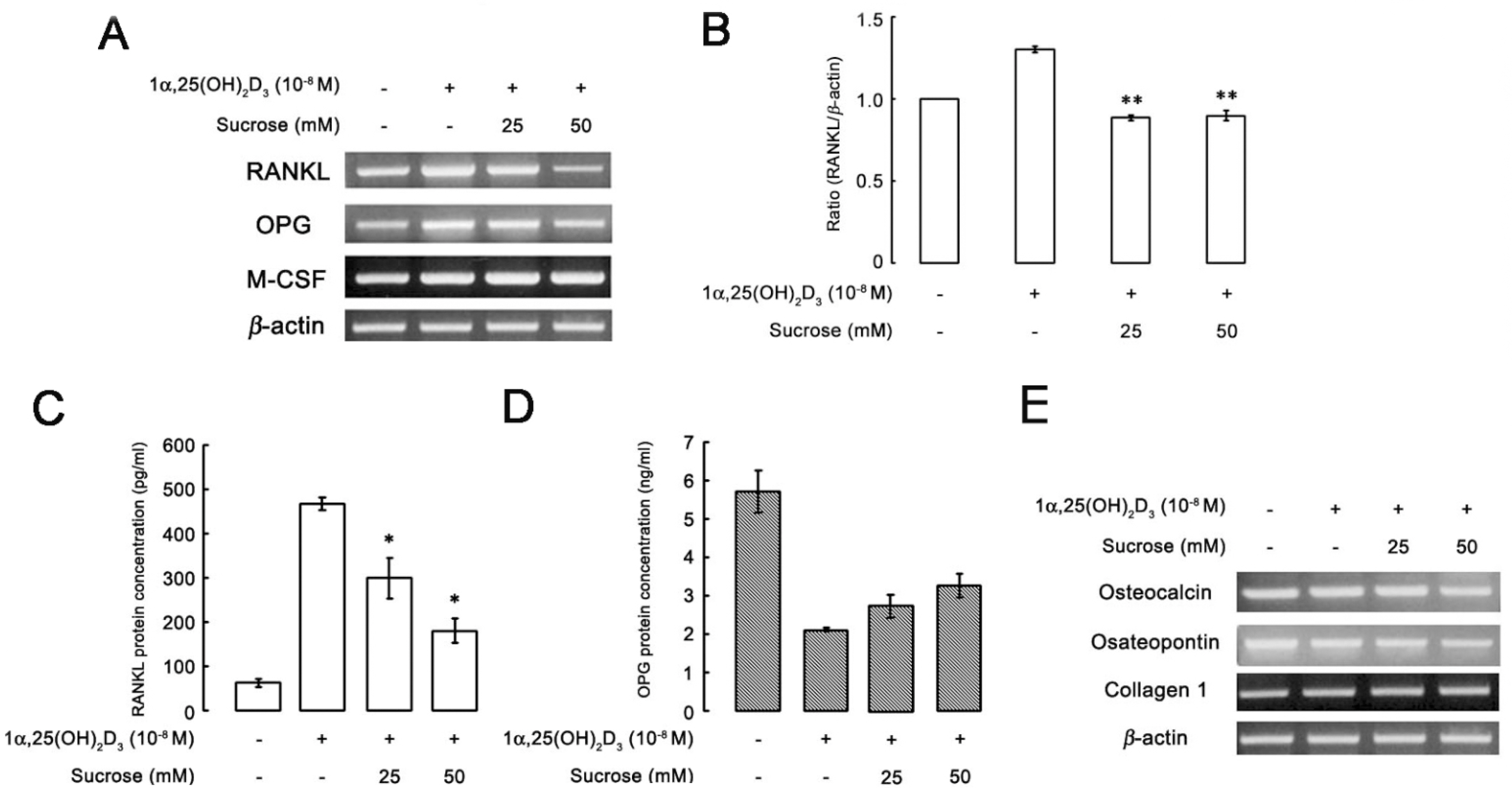
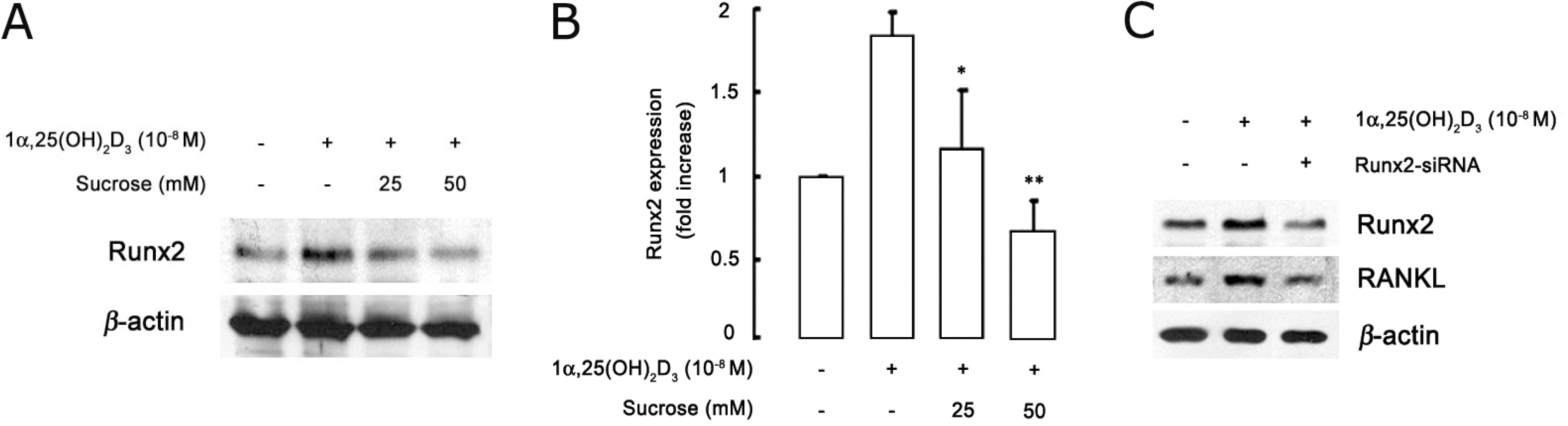
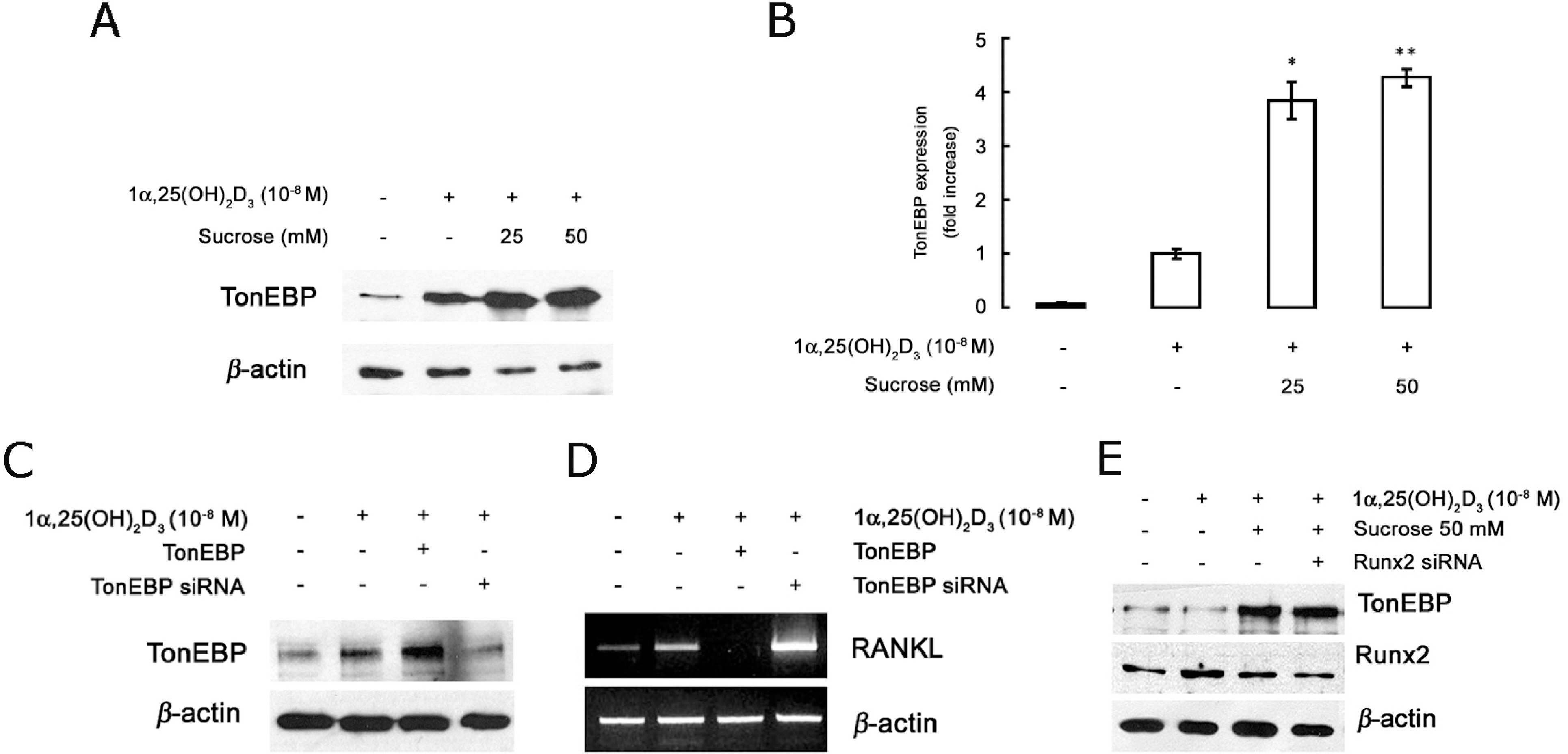
- The hyperosmotic stimulus is regarded as a mechanical factor for bone remodeling. However, whether the hyperosmotic stimulus affects 1alpha, 25-dihydroxyvitamin D3 (1alpha,25(OH)2D3)-induced osteoclastogenesis is not clear. In the present study, the effect of the hyperosmotic stimulus on 1alpha,25(OH)2D3-induced osteoclastogenesis was investigated in an osteoblast-preosteoclast co-culture system. Serial doses of sucrose were applied as a mechanical force. These hyperosmotic stimuli significantly evoked a reduced number of 1alpha,25(OH)2D3-induced tartrate-resistant acid phosphatase-positive multinucleated cells and 1alpha,25(OH)2D3-induced bone-resorbing pit area in a co-culture system. In osteoblastic cells, receptor activator of nuclear factor kappaB ligand (RANKL) and Runx2 expressions were down-regulated in response to 1alpha,25(OH)2D3. Knockdown of Runx2 inhibited 1alpha,25(OH)2D3-induced RANKL expression in osteoblastic cells. Finally, the hyperosmotic stimulus induced the overexpression of TonEBP in osteoblastic cells. These results suggest that hyperosmolarity leads to the down-regulation of 1alpha,25(OH)2D3-induced osteoclastogenesis, suppressing Runx2 and RANKL expression due to the TonEBP overexpression in osteoblastic cells.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000; 289:1504–1508.
Article2. Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002; 2:389–406.
Article3. Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992; 13:66–80.
Article4. Takahashi M, Kushida K, Naitou K. The degree of osteoporosis in patients with vertebral fracture and patients with hip fracture: relationship to incidence of vertebral fracture. J Bone Miner Metab. 1999; 17:187–194.
Article5. Tsukii K, Shima N, Mochizuki S, Yamaguchi K, Kinosaki M, Yano K, Shibata O, Udagawa N, Yasuda H, Suda T, Higashio K. Osteoclast differentiation factor mediates an essential signal for bone resorption induced by 1 alpha, 25-dihydroxyvitamin D3, prostaglandin E2, or parathyroid hormone in the microenvironment of bone. Biochem Biophys Res Commun. 1998; 246:337–341.6. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342.
Article7. Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003; 4:638–649.
Article8. Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002; 8:147–159.
Article9. Kitazawa S, Kitazawa R. RANK ligand is a prerequisite for cancer-associated osteolytic lesions. J Pathol. 2002; 198:228–236.
Article10. Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000; 43:259–269.11. Rodan GA, Martin TJ. Role of osteoblasts in hormonal control of bone resorption–a hypothesis. Calcif Tissue Int. 1981; 33:349–351.
Article12. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998; 93:165–176.
Article13. Burgess JR, David R, Greenaway TM, Parameswaran V, Shepherd JJ. Osteoporosis in multiple endocrine neoplasia type 1: severity, clinical significance, relationship to primary hyperparathyroidism, and response to parathyroidectomy. Arch Surg. 1999; 134:1119–1123.14. Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999; 402:304–309.
Article15. Bao X, Clark CB, Frangos JA. Temporal gradient in shearinduced signaling pathway: involvement of MAP kinase, c-fos, and connexin43. Am J Physiolo Heart Circ Physiol. 2000; 278:H1598–H1605.16. Breen EC. Mechanical strain increases type I collagen expression in pulmonary fibroblasts in vitro. J Appl Physiol. 2000; 88:203–209.17. Chen NX, Ryder KD, Pavalko FM, Turner CH, Burr DB, Qiu J, Duncan RL. Ca2+ regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am J Physiol Cell Physiol. 2000; 278:C989–997.18. Klein-Nulend J, Helfrich MH, Sterck JG, MacPherson H, Joldersma M, Ralston SH, Semeins CM, Burger EH. Nitric oxide response to shear stress by human bone cells cultures is endothelial nitric oxide synthase dependent. Biochem and Biophys Res Commun. 1998; 250:108–114.19. Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone soialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005; 36:1047–1055.20. Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003; 116:1397–1408.
Article21. McGarry J, Klein-Nulend J, Prendergast PJ. The effect of cytoskeletal disruption on pulsatile fluid flow-induced nitric oxide and prostaglandin E(2) in ostocytes and ostoblast. Biochem Biophys Res Commun. 2005; 330:341–348.22. Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletla reinforcement. J Cell Biol. 2005; 171:209–215.23. Miller SS, Wolf AM, Arnaud CD. Bone cells in culture: morphologic transformation by hormones. Science. 1976; 192:1340–1343.
Article24. Bowler WB, Buckley KA, Gartland A, Hipskind RA, Bilbe G, Gallagher JA. Extracellular nucleotide signaling: a mechanism for integrating local and systemic responses in the activation of bone remodeling. Bone. 2001; 28:507–512.
Article25. Marcus R. Normal and abnormal bone remodeling in man. Annu Rev Med. 1987; 38:129–141.
Article26. Choi BK, Ohk SH, Lee HJ, Kang JH, Jeong GJ, Yoo YJ. Effects of whole cell sonicates of Treponema lecithinolyticum on osteoclast differentiation. J Periodontol. 2001; 72:1172–1177.27. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article28. Tsai TT, Danielson KG, Guttapalli A, Oguz E, Albert TJ, Shapiro IM, Risbud MV. TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J Biol Chem. 2006; 281:25416–25424.
Article29. Takatsuna H, Asagiri M, Kubota T, Oka K, Osada T, Sugiyama C, Saito H, Aoki K, Ohya K, Takayanagi H, Umezawa K. Inhibition of RANKL-induced osteoclastogenesis by (–)-DHMEQ, a novel NF-kappaB inhibitor, through downregulation of NFATc1. J Bone Miner Res. 2005; 20:653–662.30. Kitazawa R, Kitazawa S, Maeda S. Promoter structure of mouse RANKL/TRANCE/OPGL/ODF gene. Biochim Biophys Acta. 1999; 1445:134–141.
Article31. Guilak F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech. 1995; 28:1529–1541.
Article32. Tsai TT, Danielson KG, Guttapalli A, Oguz E, Albert TJ, Shapiro IM, Risbud MV. TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J Biol Chem. 2006; 281:25416–24.
Article33. Tsuzuki T, Okabe K, Kajiya H, Habu T. Osmotic membrane stretch increases cytosolic Ca2+ and inhibits bone resorption activity in rat osteoclasts. Jpn J Physiol. 2000; 50:67–76.34. Zayzafoon M, Stell C, Irwin R, McCabe LR. Extracellular glucose influences osteoblast differentiation and c-jun expression. J Cell Biochem. 2000; 79:301–310.
Article35. Geoffroy V, Kneissel M, Fournier B, Boyde A, Matthias P. High bone resorption in adult aging transgenic mice overexpressing cbfa1/runx2 in cells of the osteoblastic lineage. Mol Cell Biol. 2002; 22:6222–6233.
Article36. Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997; 89:755–764.
Article37. Notoya M, Otsuka E, Yamaguchi A, Hagiwara H. Runx-2 is not essential for the vitamin D-regulated expression of RANKL and osteoprotegerin in osteoblastic cells. Biochem Biophys Res Commun. 2004; 324:655–660.
Article38. O'Brien CA, Kern B, Gubrij I, Karsenty G, Manolagas SC. Cbfa1 does not regulate RANKL gene activity in stromal/osteoblastic cells. Bone. 2002; 30:453–462.39. Sarkadi B, Parker JC. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991; 1071:407–427.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxia Inducible Factor-1alpha Directly Induces the Expression of Receptor Activator of Nuclear Factor-kappaB Ligand in MLO-Y4 Osteocytes
- Xylitol Down-Regulates 1alpha,25-Dihydroxy Vitamin D3-induced Osteoclastogenesis via in Part the Inhibition of RANKL Expression in Osteoblasts
- In Vitro Effects of 1,25-Dihydroxyvitamin D3 on the Production of Interleukin-1alpha by Ultraviolet B Irradiation in Cultured Human Keratinocyte Cell Line HaCaT Cells
- Caveolin-1 regulates osteoclast differentiation by suppressing cFms degradation
- The Effects of 1,25-Dihydroxyvitamin D3 on Expression of IGF-I Gene and Cellular Proliferation in MC3T3-E1 Cells