Korean J Physiol Pharmacol.
2010 Jun;14(3):151-156. 10.4196/kjpp.2010.14.3.151.
The Effects of Glutamate NMDA Receptor Antagonist MK-801 on Gastrointestinal Motility after Middle Cerebral Artery Occlusion in Rats
- Affiliations
-
- 1Department of Physiology, Wonkwang University School of Medicine, and Brain Research Institute at Wonkwang University, Iksan 570-749, Korea. byungp@wku.ac.kr
- 2Department of Neurosurgery, Affiliated Hospital of Yanbian University, Yanji 133000, Jilin, China.
- KMID: 2285396
- DOI: http://doi.org/10.4196/kjpp.2010.14.3.151
Abstract
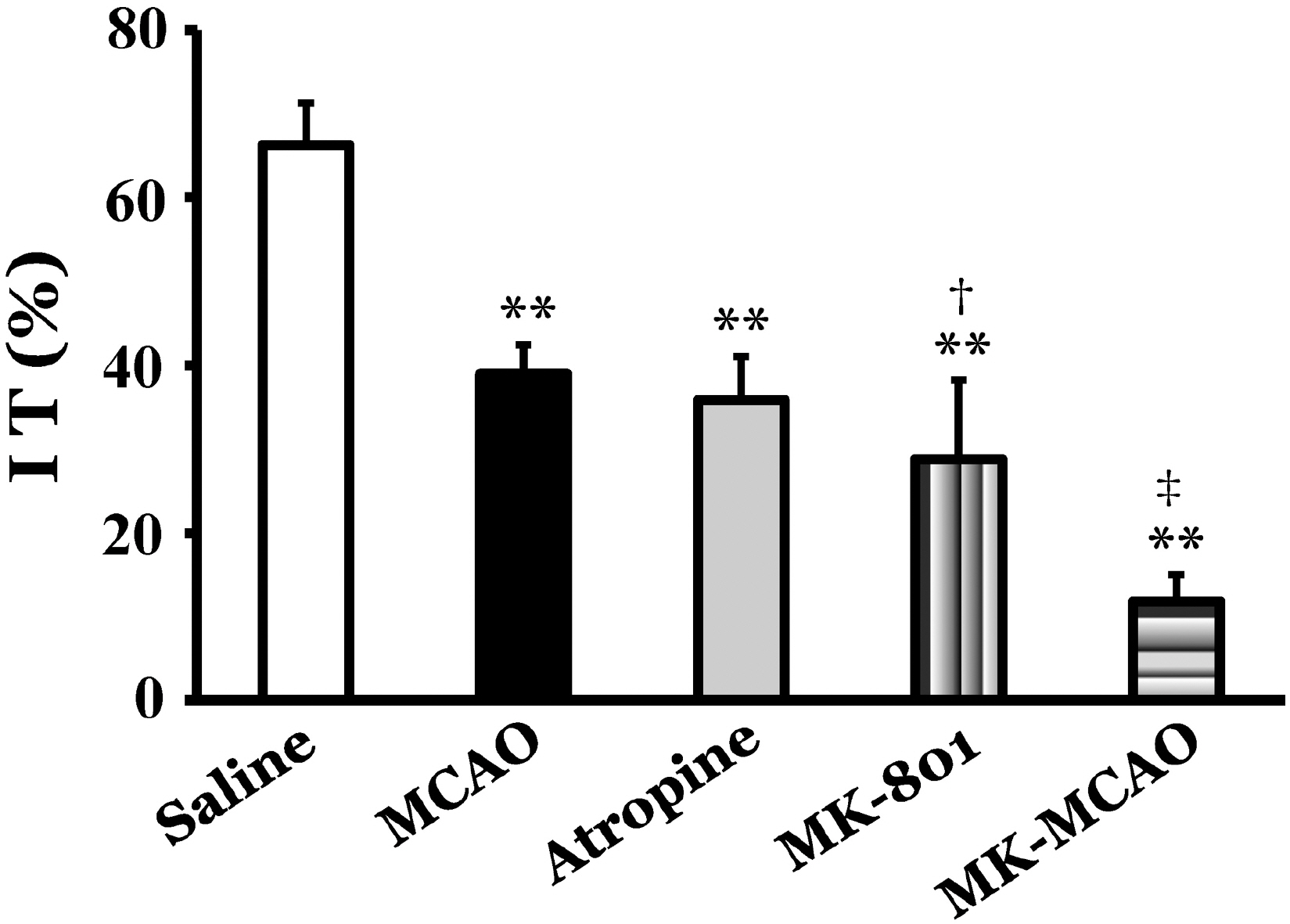
- This study was performed to investigate the role of glutamate neurotransmitter system on gastrointestinal motility in a middle cerebral artery occlusion (MCAO) model of rats. The right middle cerebral artery was occluded by surgical operation, and intestinal transit and geometric center as a parameter of gastrointestinal motility and expression of c-Fos protein in the insular cortex and cingulate cortex were measured at 2 and 12 h after MCAO. Intestinal transit was 66.3+/-7.5% and 62.3+/-5.7% 2 and 12 h after sham operation, respectively, and MCAO significantly decreased intestinal transit to 39.0+/-3.5% and 47.0+/-5.1% at 2 and 12 h after the occlusion, respectively (p<0.01). The geometric center was 5.6+/-0.4 and 5.2+/-0.9 at 2 and 12 h after sham operation, respectively, and MCAO significantly decreased geometric center to 2.9+/-0.8 and 3.0+/-0.3 at 2 and 12 h after the occlusion, respectively (p<0.01). In control animals, injection of atropine decreased intestinal transit to 35.9+/-5.2%, and injection of glutamate NMDA receptor antagonist, MK-801, decreased intestinal transit to 28.8+/-9.5%. Pretreatment with MK-801, a glutamate NMDA receptor antagonist, in the MCAO group decreased intestinal transit to 11.8+/-3.2%, which was significantly decreased compared to MCAO group (p<0.01). MCAO markedly increased the expression of c-Fos protein in the insular cortex and cingulate cortex ipsilateral to the occlusion 2 h after MCAO, and pretreatment with MK-801 produced marked reduction of c-Fos protein expression compared to MCAO group (p<0.01). These results suggest that modulation of gastrointestinal motility after MCAO might be partially mediated through a glutamate NMDA receptor system.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Cheung RT, Hachinski V. The insula and cerebrogenic sudden death. Arch Neurol. 2000; 57:1685–1688.
Article2. Cheung RT, Hachinski VC, Cechetto DF. Cardiovascular response to stress after middle cerebral artery occlusion in rats. Brain Res. 1997; 747:81–88.
Article3. Dutsch M, Burger M, Dorfler C, Schwab S, Hilz MJ. Cardiovascular autonomic function in poststroke patients. Neurology. 2007; 69:2249–2255.
Article4. Harari D, Norton C, Lockwood L, Swift C. Treatment of constipation and fecal incontinence in stroke patients: Randomized controlled trial. Stroke. 2004; 35:2549–2555.5. Bracci F, Badiali D, Pezzotti P, Scivoletto G, Fuoco U, Di Lucente L, Petrelli A, Corazziari E. Chronic constipation in hemiplegic patients. World J Gastroenterol. 2007; 13:3967–3972.6. Liu MT, Rothstein JD, Gershon MD, Kirchgessner AL. Glutamatergic enteric neurons. J Neurosci. 1997; 17:4764–4784.
Article7. Bagaev V, Aleksandrov V. Visceral-related area in the rat insular cortex. Auton Neurosci. 2006; 125:16–21.
Article8. Aleksandrov VG, Bagaev VA, Nozdrachev AD, Panteleev SS. Identification of gastric related neurons in the rat insular cortex. Neurosci Lett. 1996; 216:5–8.9. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulated cortex. Trends Cogn Sci. 2000; 4:215–222.10. Saito I, Segawa H, Shiokawa Y, Taniguchi M, Tsutsumi K. Middle cerebral artery occlusion: correlation of computed tomography and angiography with clinical outcome. Stroke. 1987; 18:863–868.
Article11. Stern RM, Koch KL, Stewart WR, Lindblad IM. Spectral analysis of tachygastria recorded during motion sickness. Gastroenterology. 1987; 92:92–97.
Article12. Cabezos PA, Vera G, Castillo M, Fernandez-Pujol R, Martin MI, Abalo R. Radiological study of gastrointestinal motor activity after acute cisplatin in the rat. Temporal relationship with pica. Auton Neurosci. 2008; 141:54–65.
Article13. Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. J Pharmacol Methods. 1981; 6:211–217.
Article14. Puig N, Davalos A, Adan J, Piulats J, Martinez JM, Castillo J. Serum amino acid levels after permanent middle cerebral artery occlusion in the rat. Cerebrovasc Dis. 2000; 10:449–454.
Article15. Laing RJ, Jakubowski J, Laing RW. Middle cerebral artery occlusion without craniectomy in rats. Which method works best? Stroke. 1993; 24:294–298.
Article16. Lee JH, Ameer AN, Choi MA, Lee MY, Kim MS, Park BR. Recovery of vestibulogastrointestinal symptoms during vestibular compensation after unilateral labyrinthectomy in rats. Otol & Neurotol. 2010; 31:241–249.
Article17. Kim MS, Jin BK, Chun SW, Lee MY, Lee SH, Kim JH, Park BR. Effect of MK801 on cfos-like protein expression in the medial vestibular nucleus at early stage of vestibular compensation in uvulonodullectomized rats. Neurosci Lett. 1997; 231:147–150.
Article18. Colivicchi F, Bassi A, Santini M, Caltagirone C. Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke. 2004; 35:2094–2098.
Article19. Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982; 212:38–52.
Article20. Schneider RJ, Friedman DP, Mishkin M. A modality-specific somatosensory area within the insula of the rhesus monkey. Brain Res. 1993; 621:16–120.
Article21. Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991; 303:355–374.
Article22. Korpelainen JT, Sotaniemi KA, Myllyla VV. Autonomic nervous system disorders in stroke. Clin Auton Res. 1999; 9:325–333.
Article23. Collaco-Moraes Y, Aspey BS, de Belleroche JS, Harrison MJ. Focal ischemia causes an extensive induction of immediate early genes that are sensitive to mk-801. Stroke. 1994; 25:1855–1860.24. Choi D. Excitotoxicity, apoptosis, and ischemic stroke. J Biochem Mol Biol. 2001; 34:8–14.25. Estus S, Zaks WJ, Freeman RS, Gruda M, Bravo R, Johnson EM Jr. Altered gene expression in neurons during programmed cell death: Identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994; 127:1717–1727.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of the Glutamate Receptor in the Transient Focal Cerebral Ischemia in Rats
- Prolonged Ischemic Cerebral Infarct in the Rat after Middle Cerebral Artery Occlusion: Part 2:Effect of the NMDA Antagonist, MK-801, Upon Ischemic Evolution
- The Effect of U-74389G and MK-801 on the Size of Brain Infarction in the Transient Focal Ischemia-Reperfusion Rat Model
- The Effects of Dexamethasone and MK-801 on Edema Formation in Middle Cerebral Artery Occlusion Model of the Rat
- Protective Effects of the NMDA-receptor Antagonist (MK-801) for the Brain Injury by Oxygen Free Radical: In the Hyperbaric Oxygen Treatment of CO Poisoned Rat