Korean J Physiol Pharmacol.
2010 Jun;14(3):133-137. 10.4196/kjpp.2010.14.3.133.
Stimulatory Effects of Ginsan on the Proliferation and Viability of Mouse Spleen Cells
- Affiliations
-
- 1Laboratory of Veterinary Pharmacology, College of Veterinary Medicine, Jeju National University, Jeju 690-756, Korea. jooh@jejunu.ac.kr
- KMID: 2285393
- DOI: http://doi.org/10.4196/kjpp.2010.14.3.133
Abstract
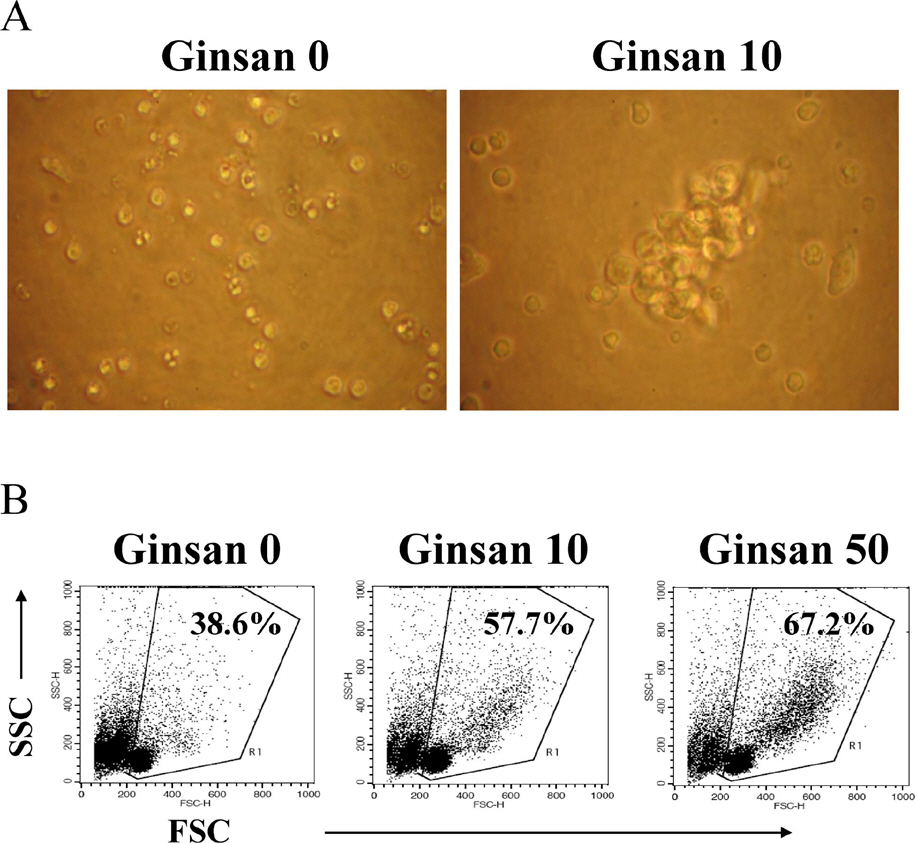
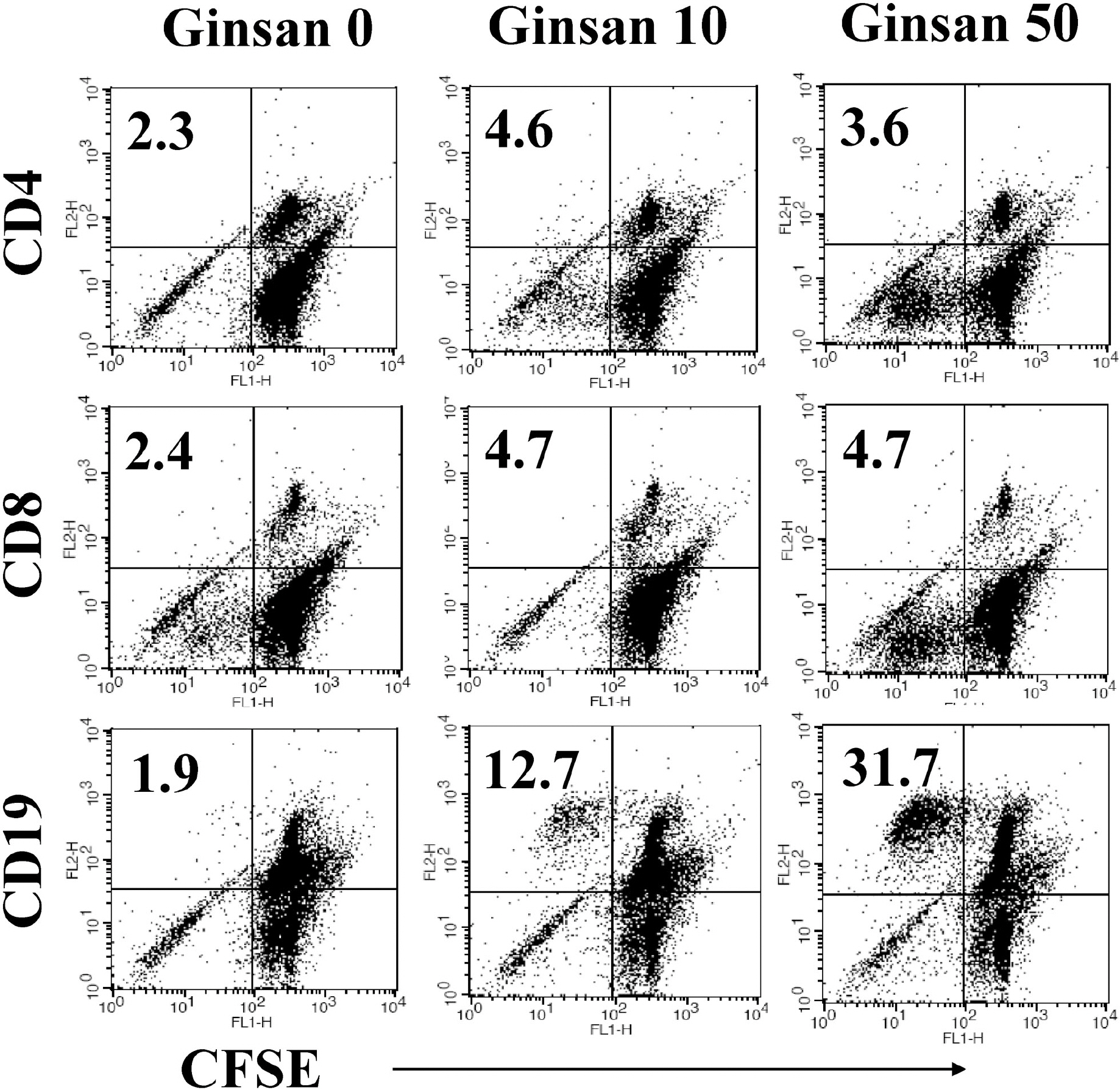
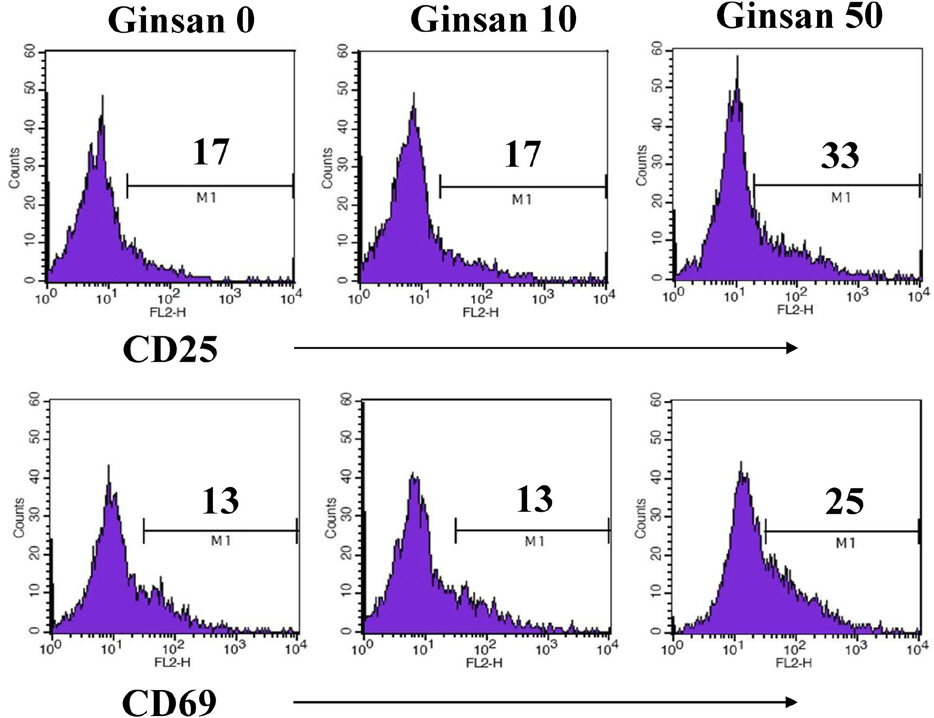
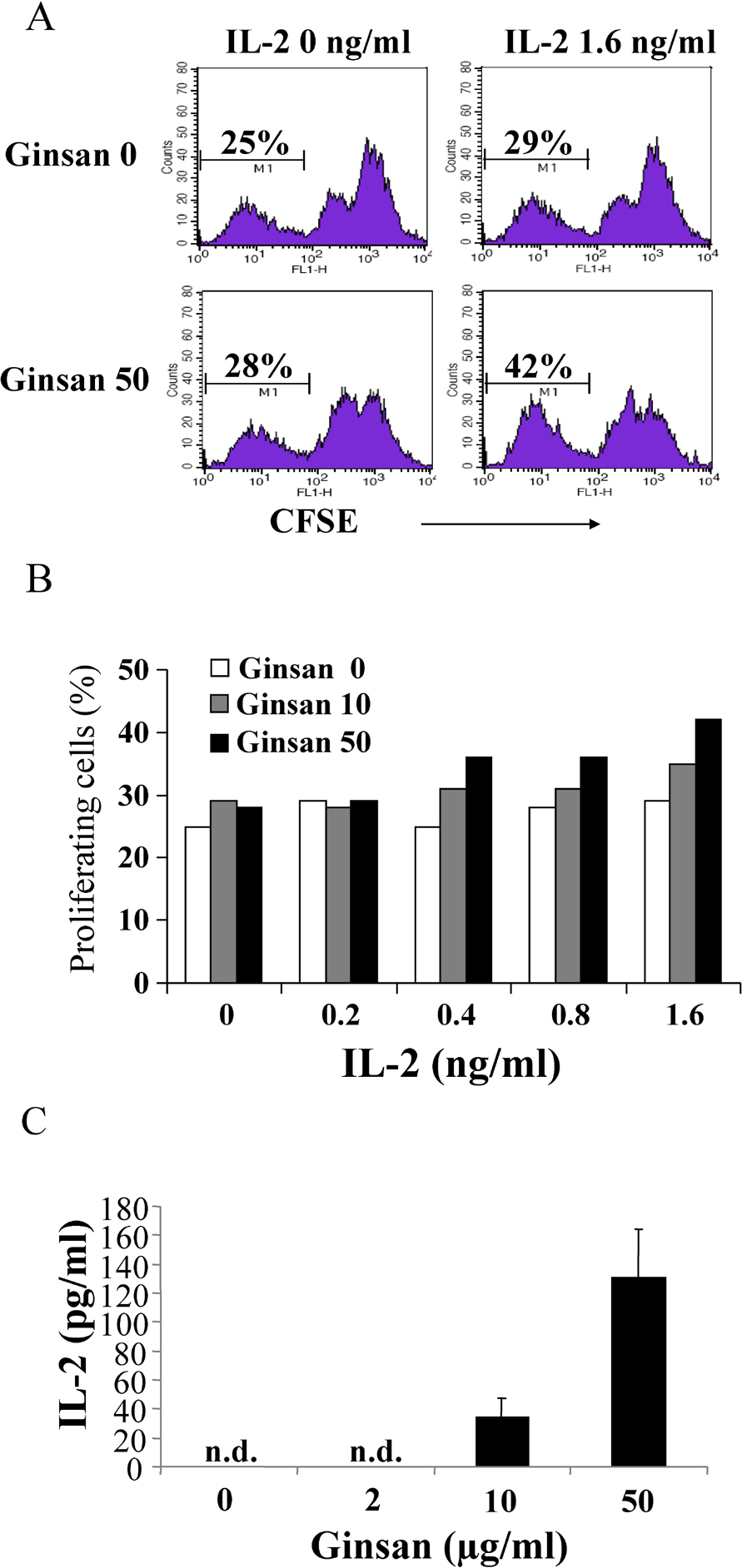
- Ginsan is an acidic polysaccharide purified from Panax ginseng, a famous oriental herb. Although a variety of biological activities of ginsan have been studied, the effects of ginsan on spleen cells are not fully elucidated. We investigated the effect of ginsan on the viability and proliferation of spleen cells. Using Cell Counting Kit-8(R) solution and trypan blue solution, we found that ginsan significantly enhanced viability and proliferation. Multiple clusters, indicating proliferation, were observed in ginsan-treated spleen cells and, carboxyfluorescein succinimidyl ester and surface marker staining assay revealed that ginsan promoted proliferation from CD19+ B cells rather than CD4+ or CD8+ T cells. In addition, ginsan decreased the percentage of late apoptotic cells. Ginsan increased the surface expression of CD25 and CD69 as well as production of interleukin-2 from spleen cells, suggesting increased activation. Taken together, these results demonstrate that ginsan increases the viability and proliferation of spleen cells via multiple mechanisms, valuable information for broadening the use of ginsan in clinical and research settings.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Immunostimulatory Effects of β-glucan Purified from
Paenibacillus polymyxa JB115 on Mouse Splenocytes
Ji-Mi Kim, Hong-Gu Joo
Korean J Physiol Pharmacol. 2012;16(4):225-230. doi: 10.4196/kjpp.2012.16.4.225.
Reference
-
References
1. Shim JY, Kim MH, Kim HD, Ahn JY, Yun YS, Song JY. Protective action of the immunomodulator ginsan against carbon tetrachloride-induced liver injury via control of oxidative stress and the inflammatory response. Toxicol Appl Pharmacol. 2010; 242:318–325.
Article2. Kim HJ, Kim MH, Byon YY, Park JW, Jee Y, Joo HG. Radio-protective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells. J Vet Sci. 2007; 8:39–44.3. Shim JY, Han Y, Ahn JY, Yun YS, Song JY. Chemoprotective and adjuvant effects of immunomodulator ginsan in cyclophosphamide-treated normal and tumor bearing mice. Int J Immunopathol Pharmacol. 2007; 20:487–497.
Article4. Ahn JY, Choi IS, Shim JY, Yun EK, Yun YS, Jeong G, Song JY. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol. 2006; 36:37–45.
Article5. Kim MH, Byon YY, Ko EJ, Song JY, Yun YS, Shin T, Joo HG. Immunomodulatory activity of ginsan, a polysaccharide of Panax ginseng, on dendritic cells. Kor J Physiol Pharmacol. 2009; 13:169–173.6. Kim KH, Lee YS, Jung IS, Park SY, Chung HY, Lee IR, Yun YS. Acidic polysaccharide from Panax ginseng, ginsan, induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with rIL-2. Planta Med. 1998; 64:110–115.7. Han SK, Song JY, Yun YS, Yi SY. Ginsan improved Th1 immune response inhibited by gamma radiation. Arch Pharm Res. 2005; 28:343–350.
Article8. Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a β-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001; 69:555–564.9. Byon YY, Kim MH, Yoo ES, Hwang KK, Jee Y, Shin T, Joo HG. Radioprotective effects of fucoidan on bone marrow cells: improvement of the cell survival and immunoreactivity. J Vet Sci. 2008; 9:359–365.
Article10. Sugrue MM, Tatton WG. Mitochondrial membrane potential in aging cells. Biol Signals Recept. 2001; 10:176–188.
Article11. Hieronymus T, Blank N, Gruenke M, Winkler S, Haas JP, Kalden JR, Lorenz HM. CD 95-independent mechanisms of IL-2 deprivation-induced apoptosis in activated human lymphocytes. Cell Death Differ. 2000; 7:538–547.
Article12. Letai A. Growth factor withdrawal and apoptosis: the middle game. Mol Cell. 2006; 21:728–730.
Article13. Fleischer A, Duhamel M, Lopez-Fernandez LA, Muñoz M, Rebollo MP, Alvarez-Franco F, Rebollo A. Cascade of transcriptional induction and repression during IL-2 deprivation-induced apoptosis. Immunol Lett. 2007; 112:9–29.
Article14. Sancho D, Gómez M, Sánchez-Madrid F. CD69 is an immunore-gulatory molecule induced following activation. Trends Immunol. 2005; 6:136–140.
Article15. Rush JS, Hodgkin PD. B cells activated via CD40 and IL-4 undergo a division burst but require continued stimulation to maintain division, survival and differentiation. Eur J Immunol. 2001; 31:1150–1159.
Article16. Quigley M, Martinez J, Huang X, Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009; 113:2256–2264.
Article17. Lee YS, Chung IS, Lee IR, Kim KH, Hong WS, Yun YS. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acidic polysaccharide ginsan isolated from Panax ginseng. Anticancer Res. 1997; 17:323–331.18. Tangye SG, Hodgkin PD. Divide and conquer: the importance of cell division in regulating B-cell responses. Immunology. 2004; 112:509–520.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunostimulatory effects of BCG-CWS on the proliferation and viability of mouse spleen cells
- Immunomodulatory Activity of Ginsan, a Polysaccharide of Panax Ginseng, on Dendritic Cells
- Induction of proliferation in resting B-cells by a factor released by activated mouse spleen cells
- Induction of apoptosis in mouse spleen cells by Ginsenoside Rp1
- Stimulatory Effect of IL-10 on Antitumor Cytolytic Activity of Murine Spleen Cells