Korean J Physiol Pharmacol.
2009 Dec;13(6):461-467. 10.4196/kjpp.2009.13.6.461.
Nitric Oxide Modulation of GABAergic Synaptic Transmission in Mechanically Isolated Rat Auditory Cortical Neurons
- Affiliations
-
- 1Department of Pharmacology, School of Dentistry, Kyungpook National University, Daegu 700-412, Korea. jjlee75@knu.ac.kr
- KMID: 2285382
- DOI: http://doi.org/10.4196/kjpp.2009.13.6.461
Abstract
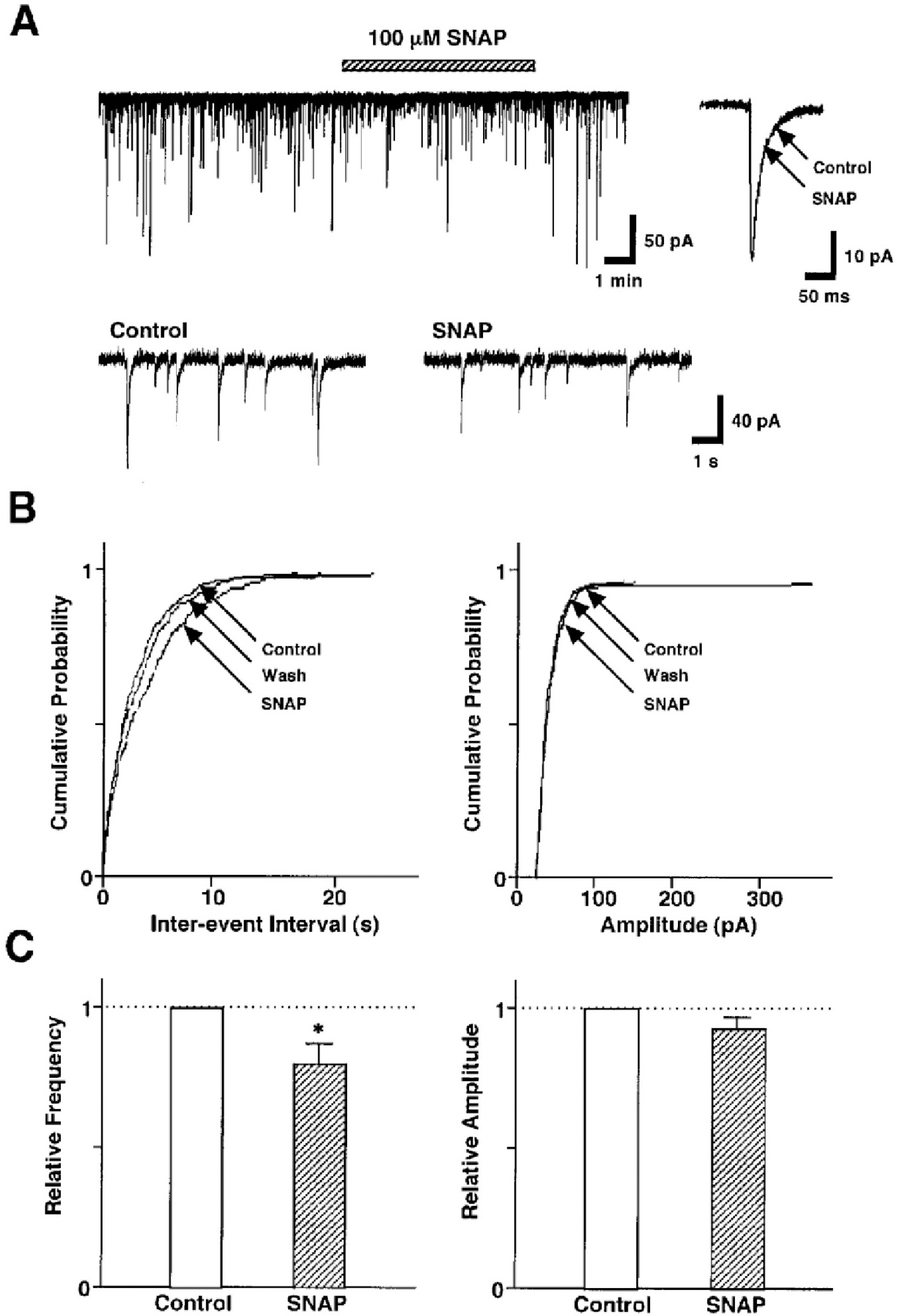
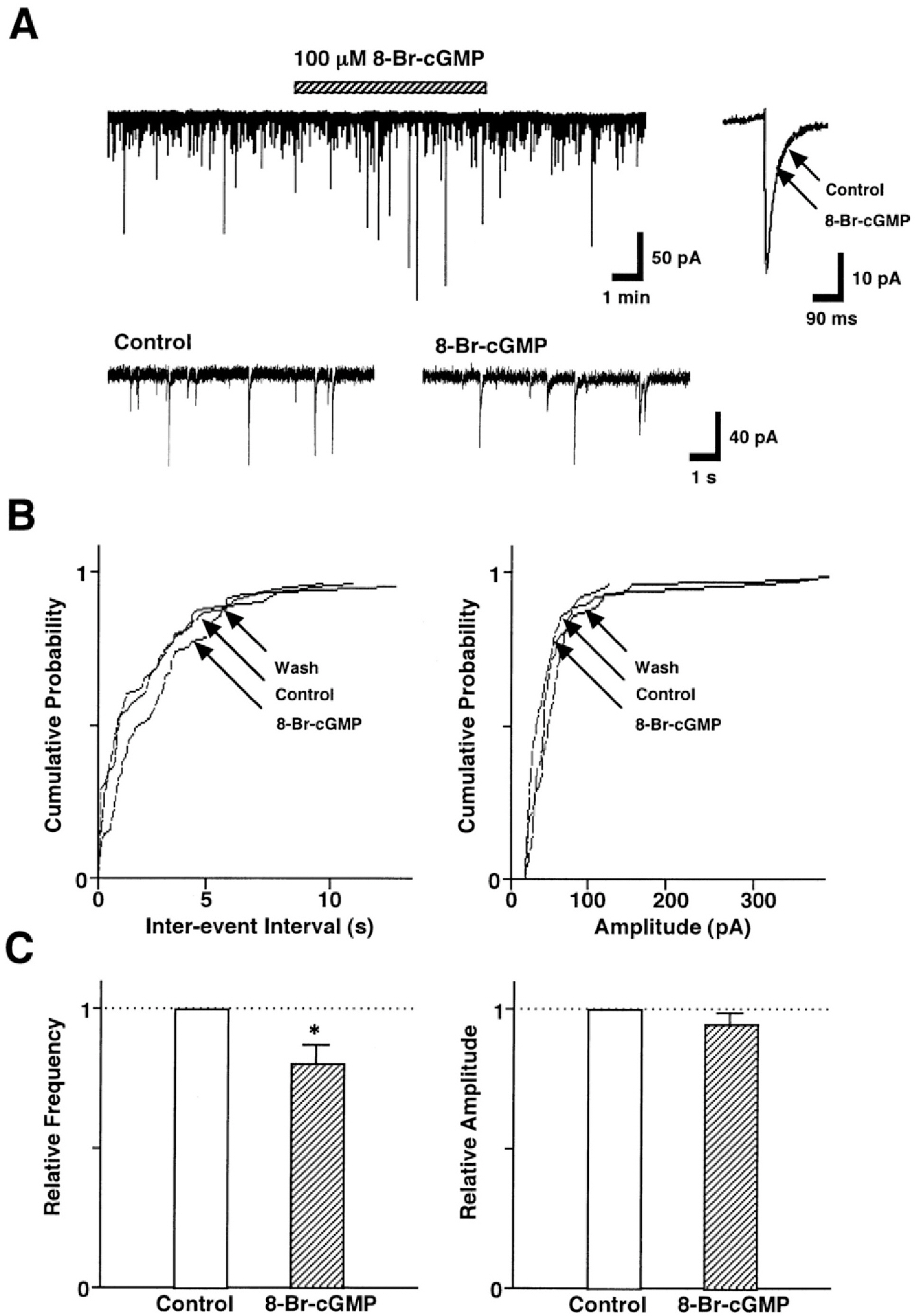
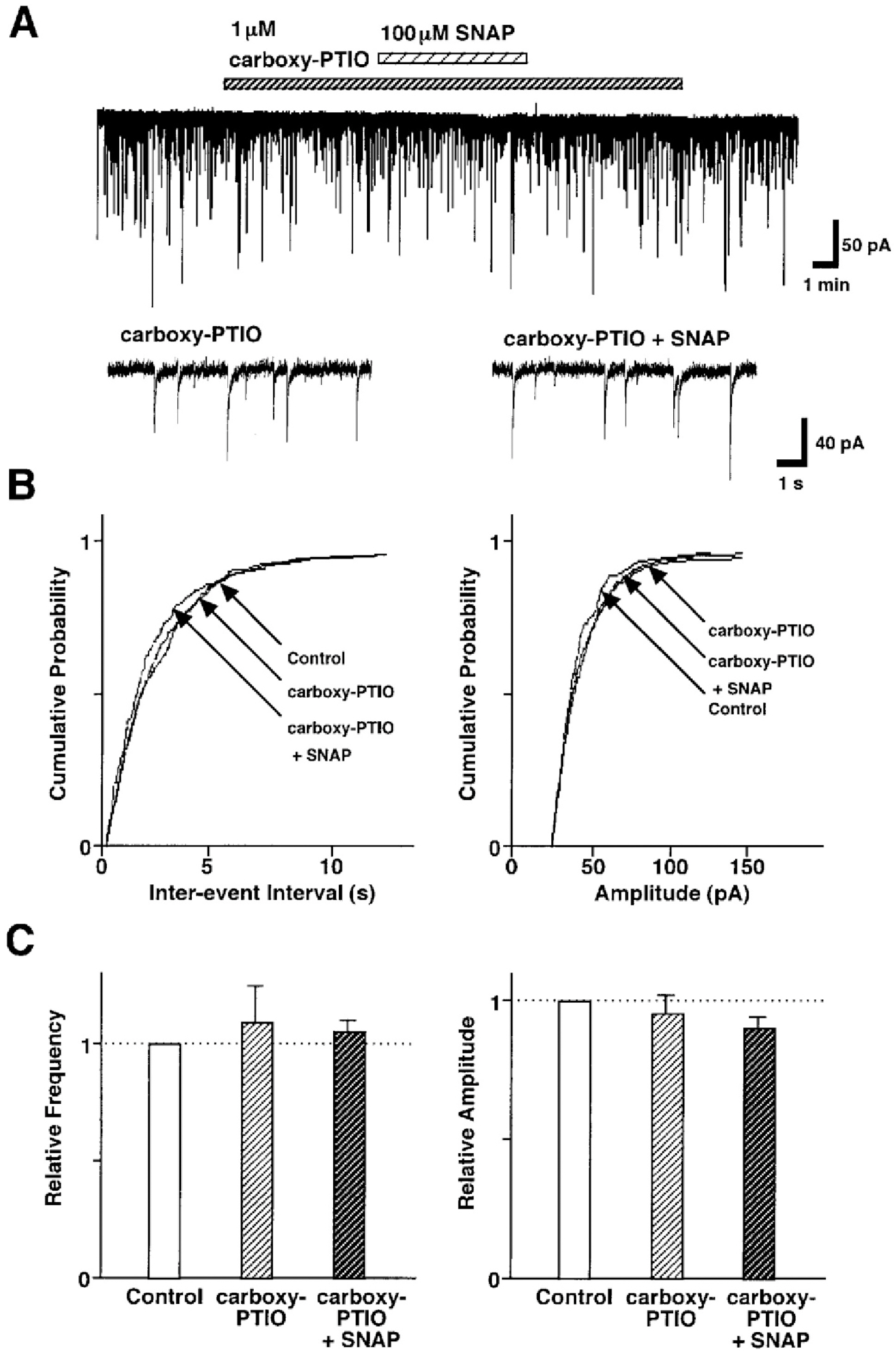
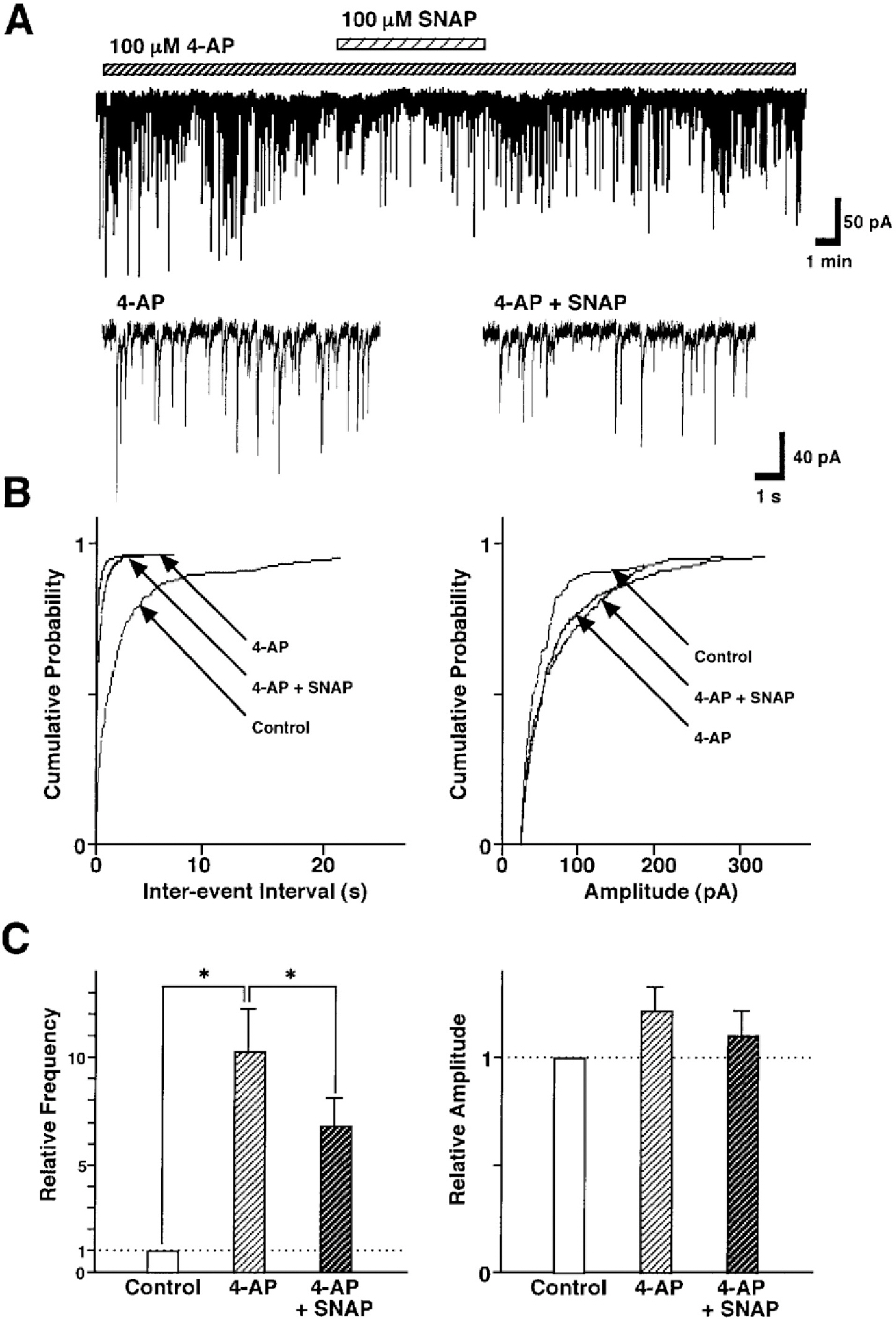
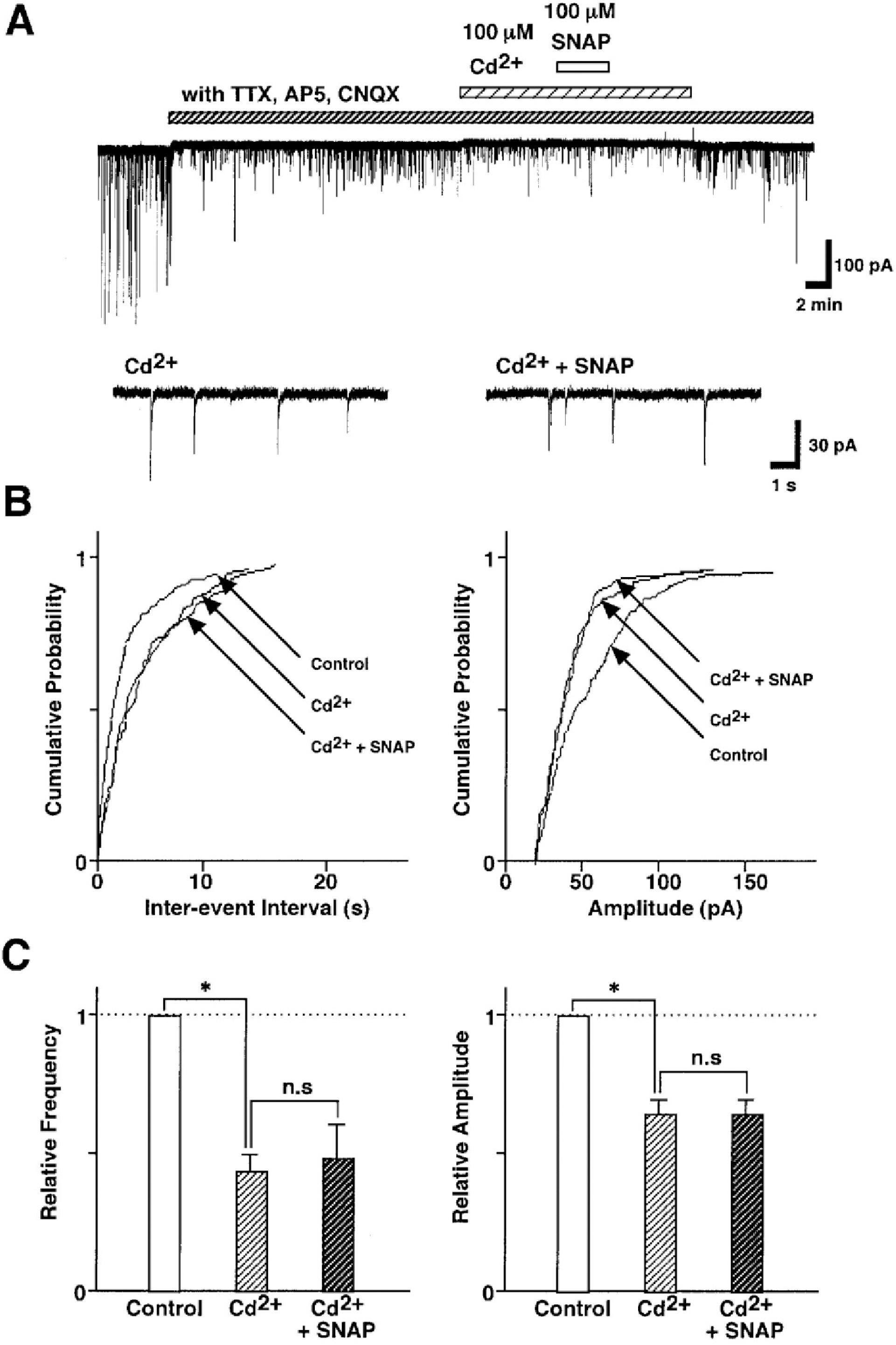
- The auditory cortex (A1) encodes the acquired significance of sound for the perception and interpretation of sound. Nitric oxide (NO) is a gas molecule with free radical properties that functions as a transmitter molecule and can alter neural activity without direct synaptic connections. We used whole-cell recordings under voltage clamp to investigate the effect of NO on spontaneous GABAergic synaptic transmission in mechanically isolated rat auditory cortical neurons preserving functional presynaptic nerve terminals. GABAergic spontaneous inhibitory postsynaptic currents (sIPSCs) in the A1 were completely blocked by bicuculline. The NO donor, S-nitroso-N-acetylpenicillamine (SNAP), reduced the GABAergic sIPSC frequency without affecting the mean current amplitude. The SNAP-induced inhibition of sIPSC frequency was mimicked by 8-bromoguanosine cyclic 3',5'-monophosphate, a membrane permeable cyclic-GMP analogue, and blocked by 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, a specific NO scavenger. Blockade of presynaptic K+ channels by 4-aminopyridine, a K+ channel blocker, increased the frequencies of GABAergic sIPSCs, but did not affect the inhibitory effects of SNAP. However, blocking of presynaptic Ca2+ channels by Cd2+, a general voltage-dependent Ca2+ channel blocker, decreased the frequencies of GABAergic sIPSCs, and blocked SNAP-induced reduction of sIPSC frequency. These findings suggest that NO inhibits spontaneous GABA release by activation of cGMP-dependent signaling and inhibition of presynaptic Ca2+ channels in the presynaptic nerve terminals of A1 neurons.
Keyword
MeSH Terms
-
4-Aminopyridine
Animals
Auditory Cortex
Benzoates
Bicuculline
gamma-Aminobutyric Acid
Guanosine
Humans
Imidazoles
Inhibitory Postsynaptic Potentials
Membranes
Neurons
Nitric Oxide
Patch-Clamp Techniques
Rats
S-Nitroso-N-Acetylpenicillamine
Synaptic Transmission
Tissue Donors
4-Aminopyridine
Benzoates
Bicuculline
Guanosine
Imidazoles
Nitric Oxide
S-Nitroso-N-Acetylpenicillamine
gamma-Aminobutyric Acid
Figure
Cited by 1 articles
-
Activation of the cGMP/Protein Kinase G Pathway by Nitric Oxide Can Decrease TRPV1 Activity in Cultured Rat Dorsal Root Ganglion Neurons
Yunju Jin, Jun Kim, Jiyeon Kwak
Korean J Physiol Pharmacol. 2012;16(3):211-217. doi: 10.4196/kjpp.2012.16.3.211.
Reference
-
References
Ahern GP., Klyachko VA., Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci. 25:510–517. 2002.
ArticleAkaike N., Harata N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn J Physiol. 44:433–473. 1994.
ArticleArnold WP., Mittal CK., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 74:3203–3207. 1977.
ArticleBoulton CL., Irving AJ., Southam E., Potier B., Garthwaite J., Collingridge GL. The nitric oxide-cyclic GMP pathway and synaptic transmission in rat hippocampal slices. Eur J Neurosci. 6:1528–1535. 1994.Bredt DS., Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 86:9030–9033. 1989.
ArticleCaspary DM., Ling L., Turner JG., Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 211:1781–1791. 2008.
ArticleCaspary DM., Raza A., Lawhorn Armour BA., Pippen J., Arneric SP. Immunocytochemical and neurochemical evidence for age-related loss of GABA in the inferior colliculus: implication for neural presbycusis. J Neurosci. 10:2363–2372. 1990.Chik CL., Liu QY., Li B., Karspinski E., Ho AK. cGMP inhibits L-type Ca2+ channel currents through protein phosphorylation in rat pinealocytes. J Neurosci. 15:3104–3109. 1995.Grassi C., D'Ascenzo M., Valente A., Battista Azzena G. Ca2+ channel inhibition induced by nitric oxide in rat insulinoma RINm5F cells. Pflügers Arch. 437:241–247. 1999.Hendry SH., Schwark HD., Jones EG., Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 7:1503–1519. 1987.
ArticleHuh YB., Park DC., Yeo SG., Cha CI. Evidence for increased NADPH-diaphorase-positive neurons in the central auditory system of the aged rat. Acta Otolaryngol. 128:648–653. 2008.
ArticleHupfer K., Jurgens U., Ploog D. The effect of superior temporal lesions on the recognition of species-specific calls in the squirrel monkey. Exp Brain Res. 30:75–87. 1977.
ArticleJaffrey SR., Snyder SH. Nitric oxide: a neural messenger. Annu Rev Cell Dev Biol. 11:417–440. 1995.
ArticleJang IS., Rhee JS., Watanabe T., Akaike N., Akaike N. Histaminergic modulation of GABAergic transmission in rat ventromedial hypothalamic neurones. J Physiol (Lond.). 534:791–803. 2001.
ArticleKlyachko VA., Ahern GP., Jackson MB. cGMP-mediated facilitation in nerve terminals by enhancement of the spike afterhyperpolarization. Neuron. 31:1015–1025. 2001.
ArticleKnight JA. The process and theories of aging. Annu Clin Lab Sci. 25:1–12. 1995.Ko GY., Kelly PT. Nitric oxide acts as a postsynaptic signaling molecule in calcium/calmodulin-induced synaptic potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 19:6784–6794. 1999.
ArticleKraus MM., Prast H. Involvement of nitric oxide, cyclic GMP and phosphodiesterase 5 in excitatory amino acid and GABA release in the nucleus accumbens evoked by activation of the hippocampal fimbria. Neuroscience. 112:331–343. 2002.
ArticleLee JJ., Cho YW., Huh YB., Cha CI., Yeo SG. Effect of nitric oxide on auditory cortical neurons of aged rats. Neurosci Lett. 447:37–41. 2008.
ArticleLiang L., Lu T., Wang X. Neural representations of sinusoidal amplitude and frequency modulations in the primary auditory cortex of awake primates. J Neurophysiol. 87:2237–2261. 2002.
ArticleLi DP., Chen SR., Finnegan TF., Pan HL. Signalling pathway of nitric oxide in synaptic GABA release in the rat paraventricular nucleus. J Physiol. 554:100–110. 2004.
ArticleLing LL., Hughes LF., Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 132:1103–1113. 2005.
ArticleManzoni O., Prezeau L., Marin P., Deshager S., Bockaert J., Fagni L. Nitric oxide induced blockade of NMDA receptors. Neuron. 8:653–662. 1992.Neff WD. The brain and hearing: auditory discriminations affected by brain leisions. Ann Otol Rhinol Laryngol. 86:500–506. 1977.Ozaki M., Shibuya I., Kabashima N., Isse T., Noguchi J., Ueta Y., Inoue Y., Shigematsu A., Yamashita H. Preferential potentiation by nitric oxide of spontaneous inhibitory postsynaptic currents in rat supraoptic neurons. J Neuroendocrinol. 12:273–281. 2000.Peinado M. Histology and histochemistry of the aging cerebral cortex; an overview. Microsc Res Tech. 43:1–7. 1998.
ArticlePineda J., Kogan JH., Aghajanian GK. Nitric oxide and carbon monoxide activate locus coeruleus neurons through a cGMP-dependent protein kinase: involvement of a nonselective cationic channel. J Neurosci. 16:1389–1399. 1996.
ArticlePrieto JJ., Peterson BA., Winer JA. Morphology and spatial distribution of GABAergic neurons in cat primary auditory cortex. J Comp Neurol. 344:349–382. 1994.Raza A., Arneric SP., Milbrandt J., Caspary DM. Age-related changes in brainstem auditory neurotransmitters: measures of GABA and acetylcholine function. Hear Res. 77:221–230. 1994.
ArticleSchreiner CE., Read HL., Sutter ML. Modular organization of frequency integration in primary auditory cortex. Annu Rev Neurosci. 23:501–529. 2000.
ArticleStamler JS., Toone EJ., Lipton SA., Sucher NJ. (S)NO signals: translocation, regulation, and a consensus motif. Neuron. 18:691–696. 1997.
ArticleTewari K., Simard JM. Sodium nitroprusside and cGMP decrease Ca2+ availability in basilar artery smooth muscle cells. Pflügers Arch. 433:304–311. 1997.Tohse N., Sperelakis N. cGMP inhibits the activity of single calcium channels in embryonic chick heart cells. Circ Res. 69:325–331. 1991.
ArticleWei JY., Ethan J., Cohen D., Daw NW., Barnstable CJ. cGMP-induced presynaptic depression and postsynaptic facilitation at glutamatergic synapses in visual cortex. Brain Res. 927:42–54. 2002.
ArticleWu LG., Saggau P. Pharmacological identification of two types of presynaptic voltage-dependent calcium channels at CA3-CA1 synapses of the hippocampus. J Neurosci. 14:5613–5622. 1994.Yang Q., Chen SR., Li DP., Pan HL. KV1.1/1.2 channels are downstream effectors of nitric oxide on synaptic GABA release to preautonomic neurons in the paraventricular nucleus. Neuroscience. 149:315–327. 2007.
ArticleZagotta WN., Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 19:235–263. 1996.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distribution and synaptic organization of nitric oxide synthase immunoreactive neurons in the rat olfactory
- Modulation of Presynaptic GABA Release by Oxidative Stress in Mechanically-isolated Rat Cerebral Cortical Neurons
- Effect of Long-Term Food Restriction on Nitric Oxide Synthase-Positive Neurons in Rat Cerebral Cortex
- Distribution of Neuropeptide Y and Nitric Oxide Synthase Containing Neurons in the Cerebral Cortex of Aged Rats
- Seizure-Related Change of NADPH-diaphorase and Calbindin D28k Positive Neurons in the Cerebral Cortex of Rats