Korean J Physiol Pharmacol.
2008 Jun;12(3):111-115. 10.4196/kjpp.2008.12.3.111.
(-)-Epigallocatechin Gallate Inhibits the Pacemaker Activity of Interstitial Cells of Cajal of Mouse Small Intestine
- Affiliations
-
- 1Department of Rehabilitation, College of Medicine, Chosun University, Gwangju 501-759, Korea.
- 2Department of Radiology, Gacheon University Gil Medical Center, Incheon 405-760, Korea.
- 3Department of Psychiatry, College of Medicine, Chosun University, Gwangju 501-759, Korea.
- 4Department of Physiology, College of Medicine, Chosun University, Gwangju 501-759, Korea. jyjun@chosun.ac.kr
- KMID: 2285351
- DOI: http://doi.org/10.4196/kjpp.2008.12.3.111
Abstract
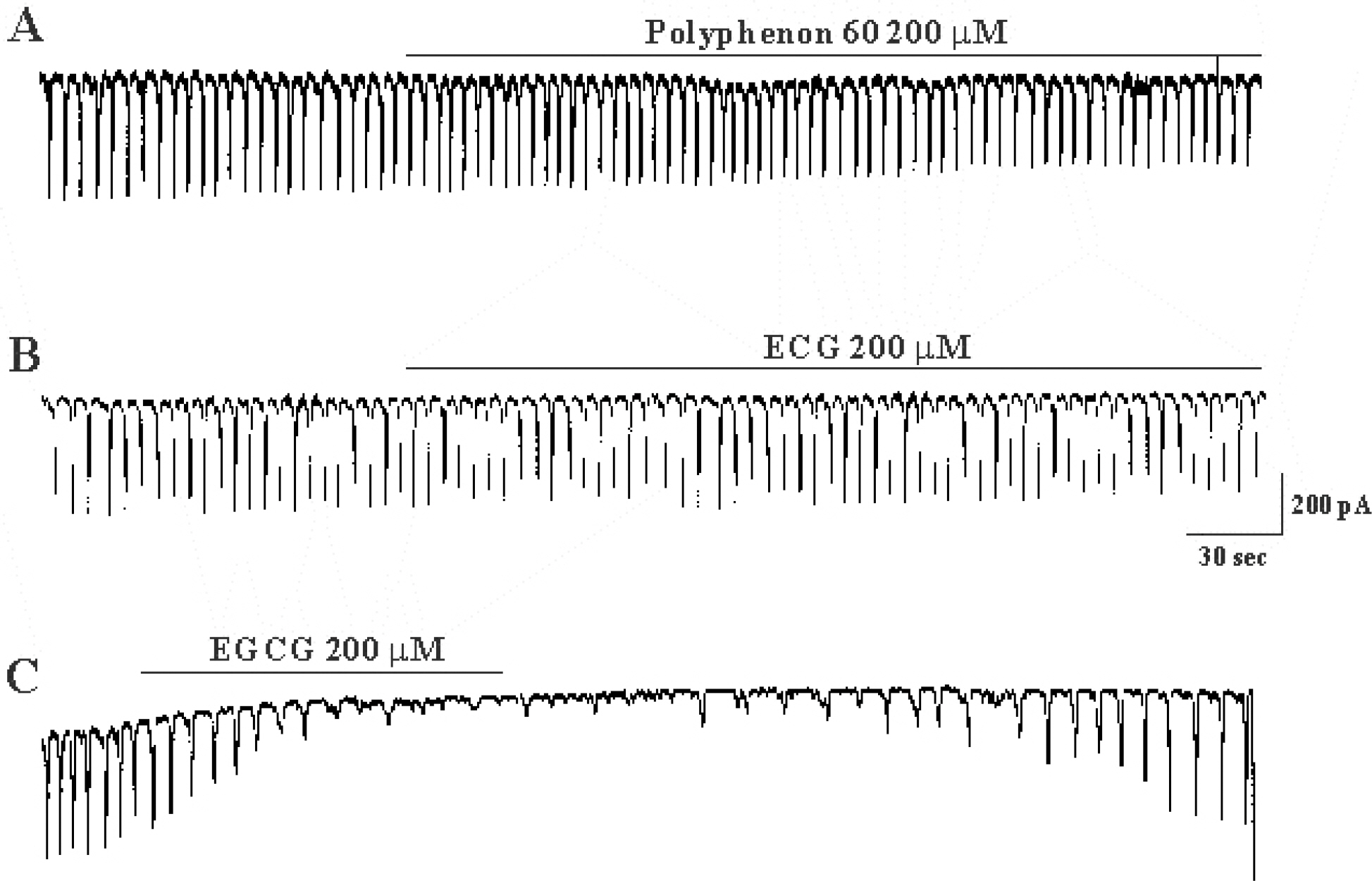
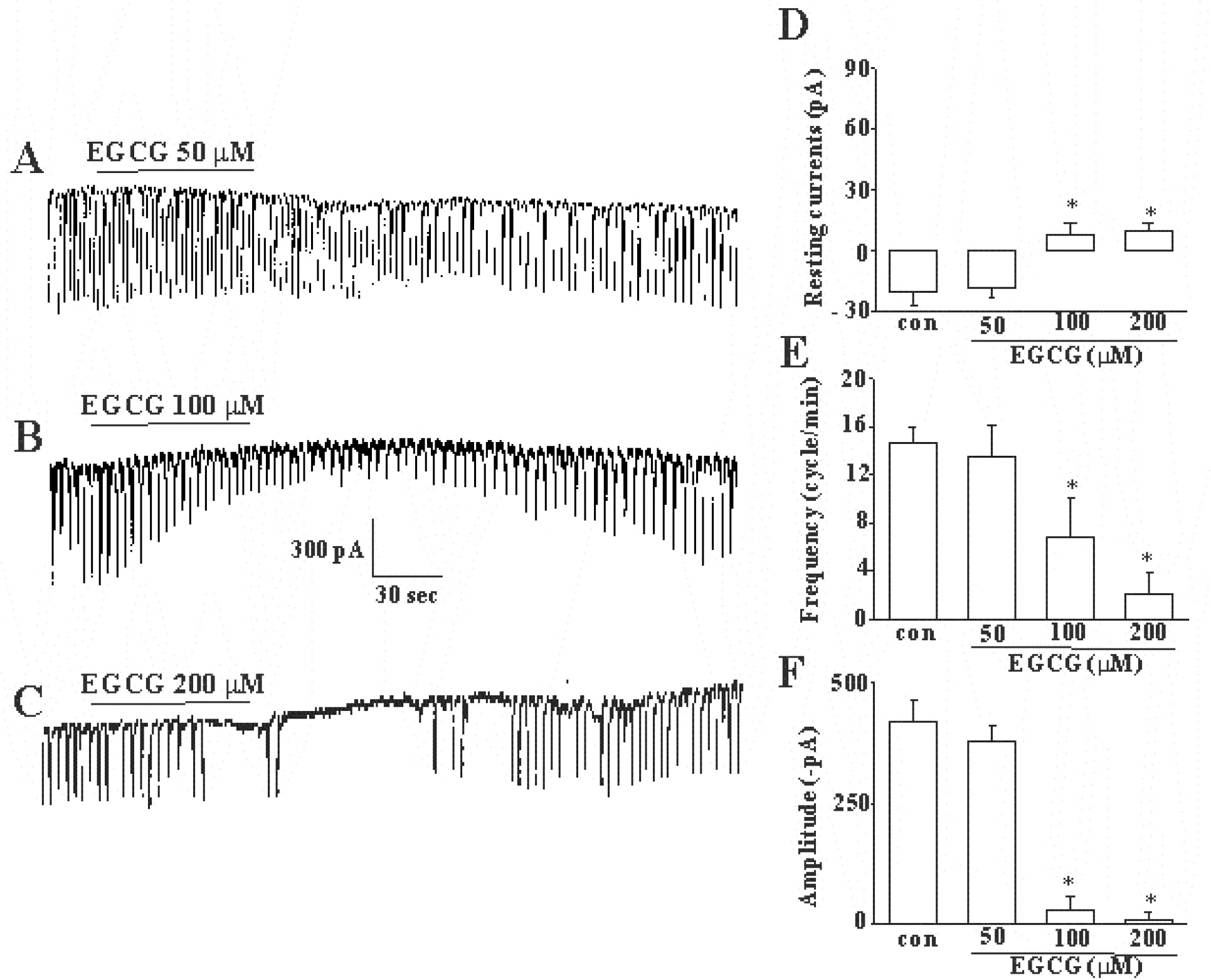
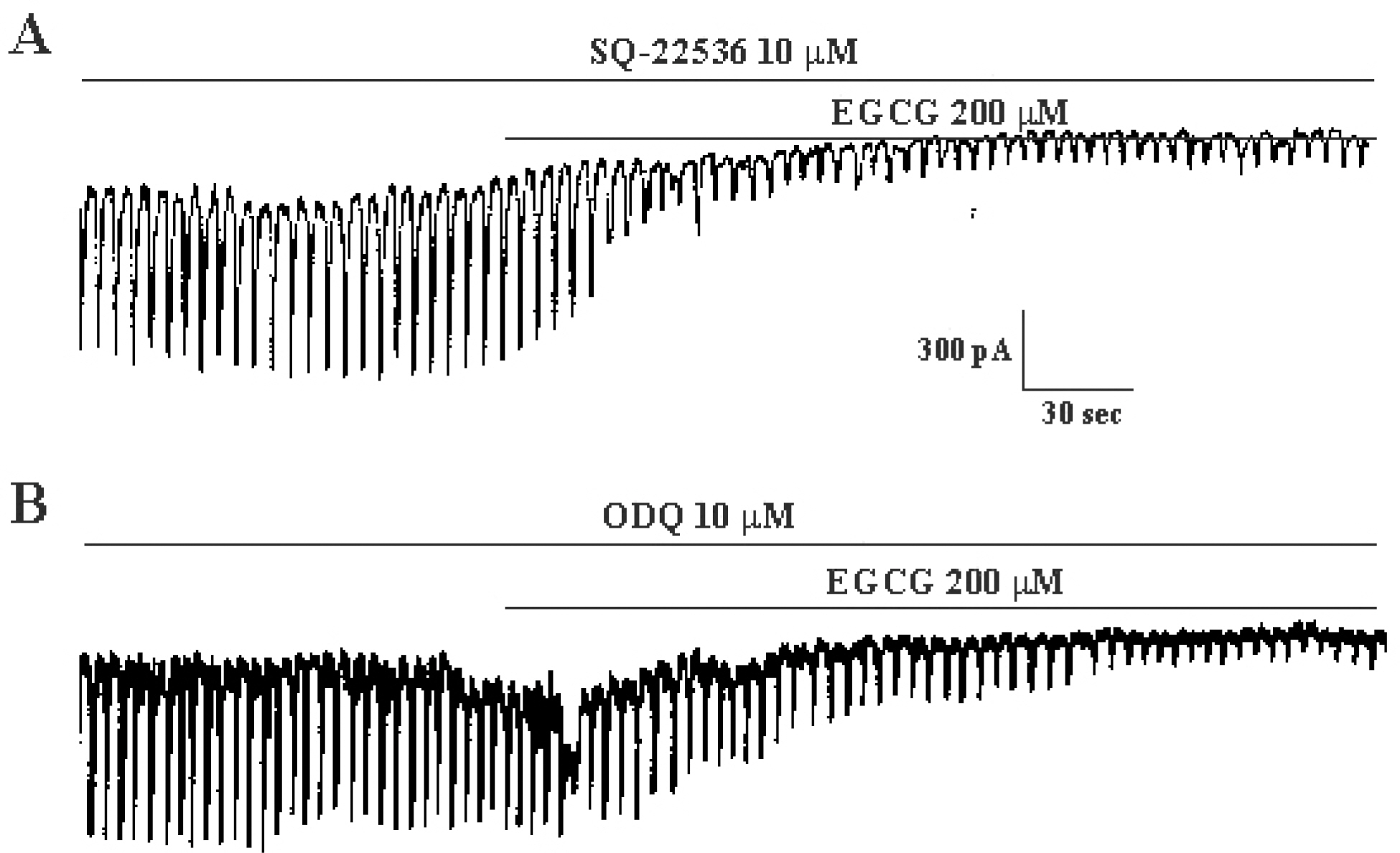
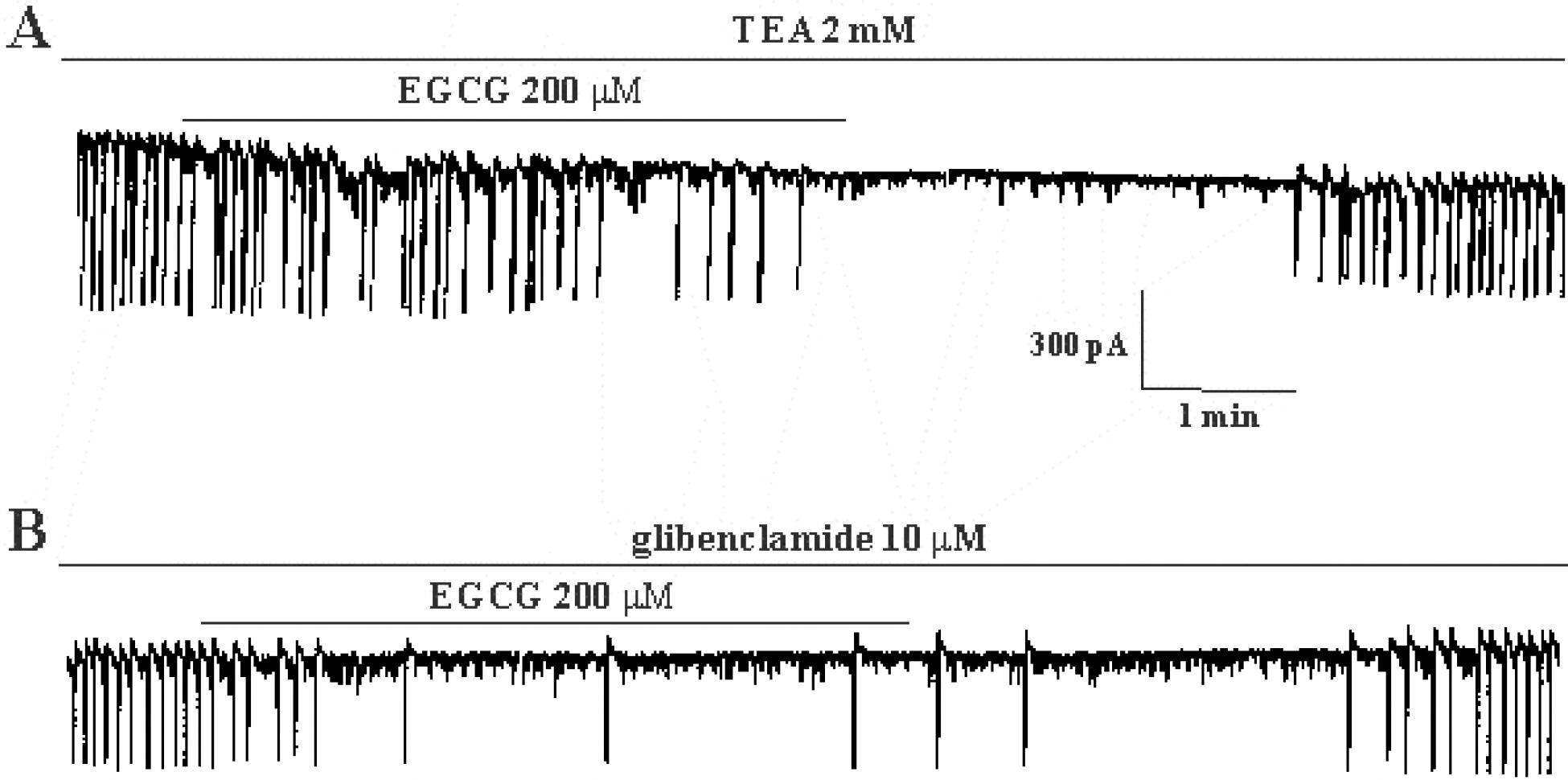
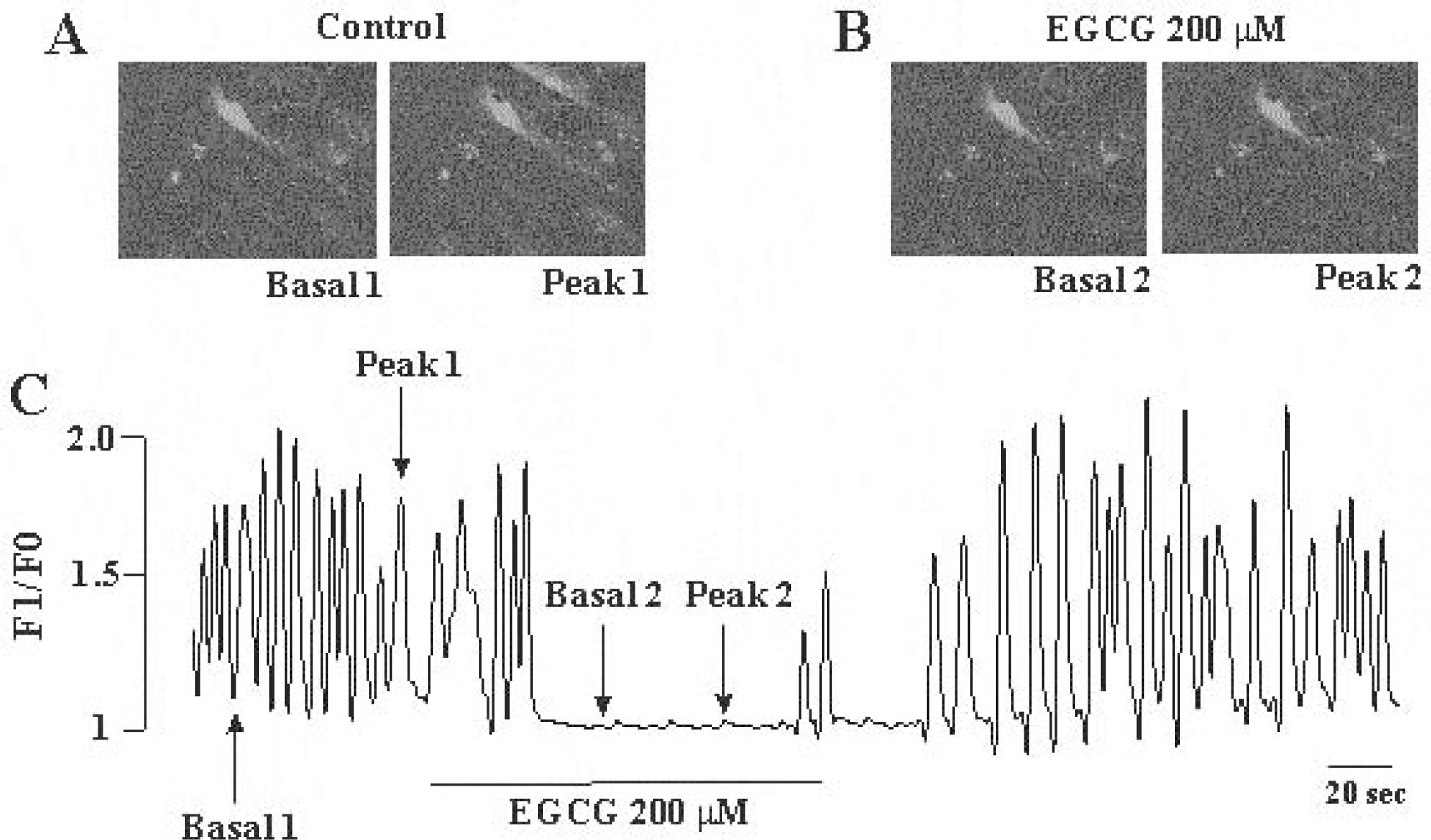
- The effects of (-)-epigallocatechin gallate (EGCG) on pacemaker activities of cultured interstitial cells of Cajal (ICC) from murine small intestine were investigated using whole-cell patch-clamp technique at 30degrees C and Ca2+ image analysis. ICC generated spontaneous pacemaker currents at a holding potential of -70 mV. The treatment of ICC with EGCG resulted in a dose-dependent decrease in the frequency and amplitude of pacemaker currents. SQ-22536, an adenylate cyclase inhibitor, and ODQ, a guanylate cyclase inhibitor, did not inhibit the effects of EGCG. EGCG-induced effects on pacemaker currents were not inhibited by glibenclamide, an ATP-sensitive K+ channel blocker and TEA, a Ca2+-activated K+ channel blocker. Also, we found that EGCG inhibited the spontaneous [Ca2+]i oscillations in cultured ICC. In conclusion, EGCG inhibited the pacemaker activity of ICC and reduced [Ca2+]i oscillations by cAMP-, cGMP-, ATP-sensitive K+channel-independent manner.
Keyword
MeSH Terms
Figure
Reference
-
Alvarez E., Campos-Toimil M., Hustiniano-Basaran H., Lugnier C., Orallo F. Study of the mechanisms involved in the vasorelaxation induced by (–)-epigallocatechin-3-gallate in rat aorta. Br J Pharm. 147:269–280. 2006.Alvarez-Castro E., Campos-Toimil M., Orallo F. (–)-epigallocatechin-3-gallate induces contraction of the rat aorta by a calcium influx-dependent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 369:496–506. 2004.Aoyama M., Yamada A., Wang J., Ohya S., Furuzono S., Goto T., Hotta S., Ito Y., Matsubara T., Shimokata K., Chen SR., Imaizumi Y., Nakayama S. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC in HEK293 cells. J Cell Sci. 117:2813–2825. 2004.Baek WK., Jang BC., Lim JH., Kwon TK., Lee HY., Cho CH., Kim DK., Shin DH., Park JG., Lim JG., Bae JH., Bae JH., Yoo SK., Park WK., Song DK. Inhibitory modulation of ATP-sensitive potassium channels by gallate-ester moiety of (–)-epigallocatechin-3-gallate. Biochem Pharm. 70:1560–1567. 2005.
ArticleCeregrzyn M., Kuwahara A. The effect of epigallocatechin gallate on intestinal motility in mice. Environ Health Prev Med. 8:47–51. 2003.
ArticleChen ZY., Law WI., Yao XQ., Lau CW., Ho WK., Huang Y. Inhibitory effects of purified green tea epicatechins on contraction and proliferation of arterial smooth muscle cells. Acta Pharmacologica Sinica. 21:835–840. 2000.Choi S., Chang IY., Yeum CH., You HJ., Park JS., Jeong HS., So I., Kim KW., Jun JY. Activating of ATP-dependent K+ channels comprised of Kir 6.2 and SUR2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine. Cell Physiol Biochem. 18:187–198. 2006.Di Carlo G., Autore G., Izzo AA., Maiolino P., Mascolo N., Viola P., Diurno MV., Capasso F. Inhibition of intestinal motility and secretion by flavonoids in mice and rats: structure-activity relationships. J Pharm Pharmacol. 45:1054–1059. 1993.
ArticleGraham HN. Green tea composition, consumption, and polyphenol chemistry. Prevent Med. 21:334–350. 1992.
ArticleHara Y. Green Tea; Health benefits and applications. Marcel Dekker, Inc;New York: 2001.Homma T., Hirai K., Hara Y., Katayama Y. Tea catechin, (–)-epigallocatechin gallate, causes membrane depolarizations of myenteric neurons in the guinea-pig small intestine. Neurosci Lett. 309:93–96. 2001.
ArticleHuang Y., Zhang A., Lau CW., Chen ZY. Vasorelaxant effects of purified green tea epicatechin derivatives in rat mesenteric artery. Life Sci. 63:275–283.
ArticleHuizinga JD., Thuneberg L., Kluppel M., Malysz J., Mikkelsen HB., Bernstein A. W/kit gene required for intestinal pacemaker activity. Nature. 373:347–352. 1995.Jayaraj AP., Lewin MR., Tovey FI., Kitler ME., Clark CG. The protective effect of Meciadanol (O-methyl-3(+)-catechin) on experimental ulceration. Eur J Pharmacol. 147:265–271. 1988.
ArticleKoh SD., Sanders KM., Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the mouse small intestine. J Physiol. 513:203–213. 1998.Katayama Y., Homma T., Hara Y., Hara Y., Hirai K. Tea catechin, (–)-epigallocatechin gallate, facilitates cholinergic ganglion transmission in the myenteric plexus of the guinea-pig small intestine. Neurosci Lett. 319:63–66. 2002.
ArticleKonturek SJ., Kitler ME., Brzozowski T., Radecki T. Gastric protection by meciadanol. Dig Dis Sci. 31:847–852. 1986.
ArticleMatsuzaki T., Hara Y. Antioxidative activity of tea leaf catechins. Nippon Nogeikagaku Kaishi. 59:129–134. 1985.
ArticleMinowa T., Miwa S., Kobayashi S., Enoki T., Zhang XF., Komuro T., Iwamuro Y., Masaki T. Inhibitory effect of nitrovasodilators and cyclic GMP on ET-1-activated Ca2+ permeable nonselective cation channel in rat aortic smooth muscle cells. Br J Pharm. 271:515–522. 1990.Orallo F. Regulation of cytosolic calcium levels in vascular smooth muscle. Pharm Therap. 69:153–171. 1996.
ArticleOrlov SN., Tremblay J., Hamet P. cAMP signaling inhibits dihydropyridine-sensitive Ca2+ influx in vascular smooth muscle cells. Hypertension. 27:774–780. 1996.Park CG., Kim MY., Kim JS., Choi S., Yeum CH., Parajuli SP., Park JS., Jeong HS., So I., Kim KW., Jun JY. Inhibition of pacemaker currents by nitric oxide via activation of ATP-sensitive K+ channels in cultured interstitial cells of Cajal from mouse small intestine. Naunyn-Schmiedeberg Arch Pharmacol. 376:175–184. 2007.Rodrigo GC., Standen NG. ATP-sensitive potassium channels. Curr Pharam Design. 11:1915–1940. 2005.
ArticleSanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 111:492–515. 1996.
ArticleShuttleworth CW., Xue C., Ward SM., de Vent J., Sanders KM. Immunohisochemical localization of 3',5'-cyclic guanosine monophosphate in the canine proximal colon: response to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience. 56:513–522. 1993.Suzuki H., Takano H., Yamamoto Y., Komuro T., Saito M., Kato K., Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol triphosphate receptor. J Physiol. 525:105–111. 2000.Thomsen L., Robinson TL., Lee JCF., Farraway L., Hughes MJH., Andrews DW., Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nature Med. 4:848–51. 1998.Thuneberg L. Interstitial cells of Cajal: Intestinal pacemakers? Adv Anat Embryol Cell Biol. 71:1–130. 1982.Tokutomi N., Maeda H., Tokutomi Y., Sato D., Sugita M., Nishikawa S., Nishikawa S., Nakao J., Imamura T., Nishi K. Rhythmic Cl-current and physiological roles of the intestinal c-kit-positive cells. Pflugers Arch. 431:169–177. 1995.Viswanathan S., Thirugnana Sambantham P., Bapna JS., Kameswaran L. Flavonoid-induced delay in the small intestinal transit: possible mechanism of action. Arch Int Pharmacodyn Ther. 270:151–157. 1984.Ward SM., Ordog T., Koh SD., Baker SA., Jun YT., Amberg G., Monaghan K., Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 525:355–361. 2000.
ArticleWard SM., Burns AJ., Torihashi S., Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 480:91–102. 1994.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Capsaicin Inhibits the Spontaneous Pacemaker Activity in Interstitial Cells of Cajal From the Small Intestine of Mouse
- Interplay of Hydrogen Sulfide and Nitric Oxide on the Pacemaker Activity of Interstitial Cells of Cajal from Mouse Small Intestine
- Effects of ATP on Pacemaker Activity of Interstitial Cells of Cajal from the Mouse Small Intestine
- Inhibition of Pacemaker Activity of Interstitial Cells of Cajal by Hydrogen Peroxide via Activating ATP-sensitive K(+) Channels
- Modulation of Pacemaker Ca2+ Activity by Serotonin in the Cultured Interstitial Cells of the Cajal Clusters Isolated from Mouse Small Intestine