Korean J Physiol Pharmacol.
2008 Jun;12(3):89-94. 10.4196/kjpp.2008.12.3.89.
White Matter Damage and Hippocampal Neurodegeneration Induced by Permanent Bilateral Occlusion of Common Carotid Artery in the Rat: Comparison between Wistar and Sprague-Dawley Strain
- Affiliations
-
- 1Department of Pharmacology, Cell Death Disease Research Center, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea. syk@catholic.ac.kr
- KMID: 2285348
- DOI: http://doi.org/10.4196/kjpp.2008.12.3.89
Abstract
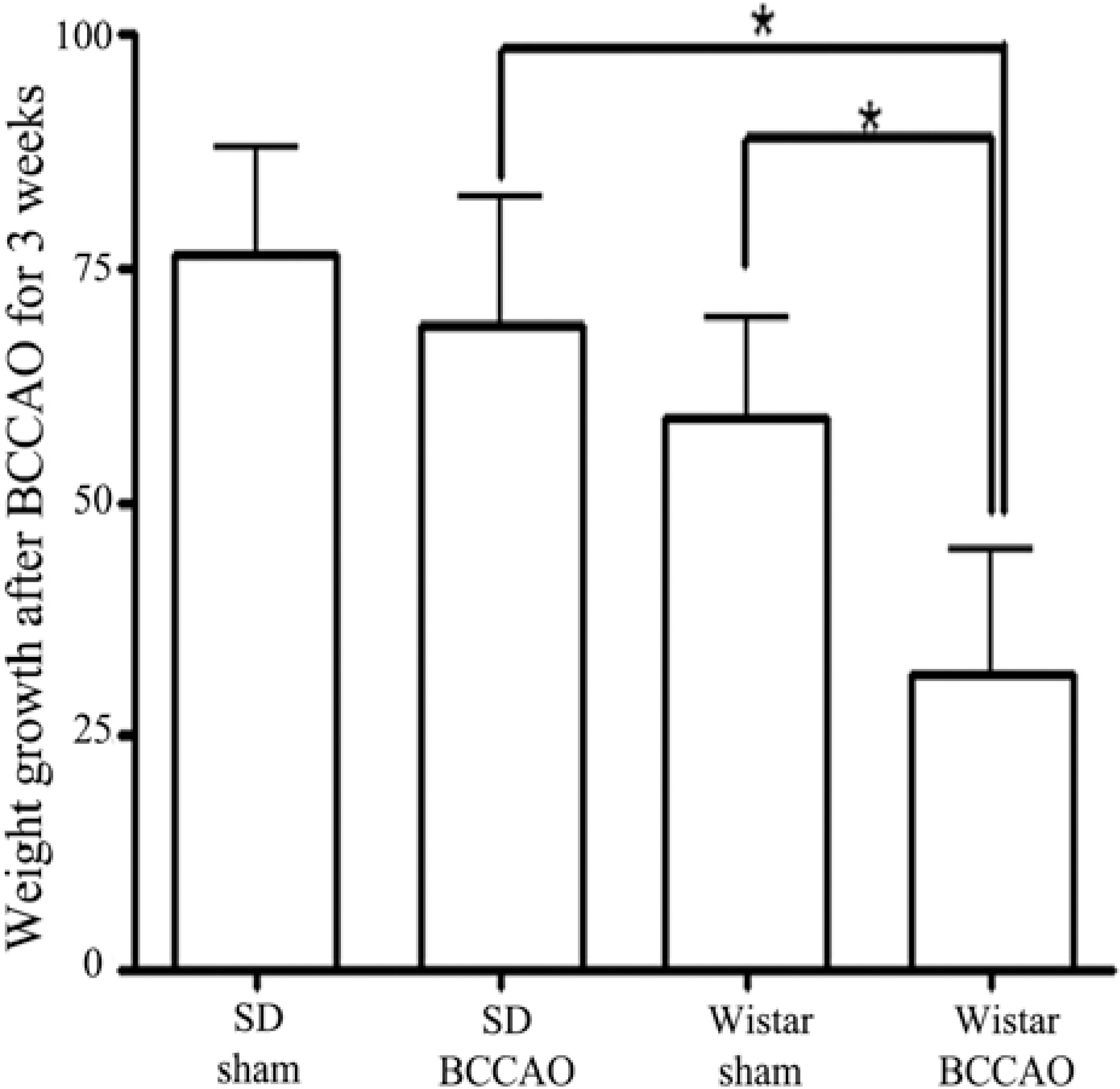
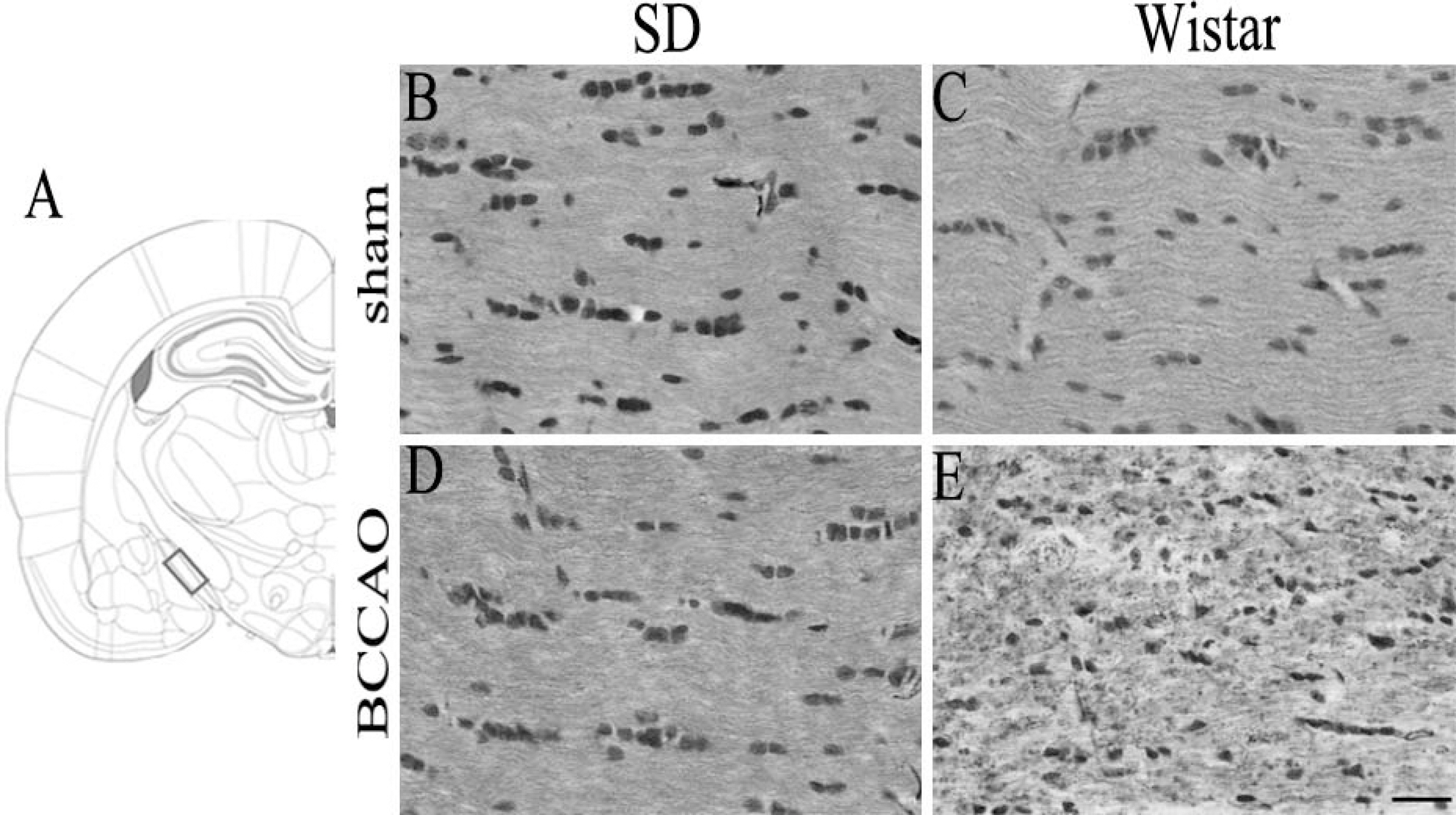
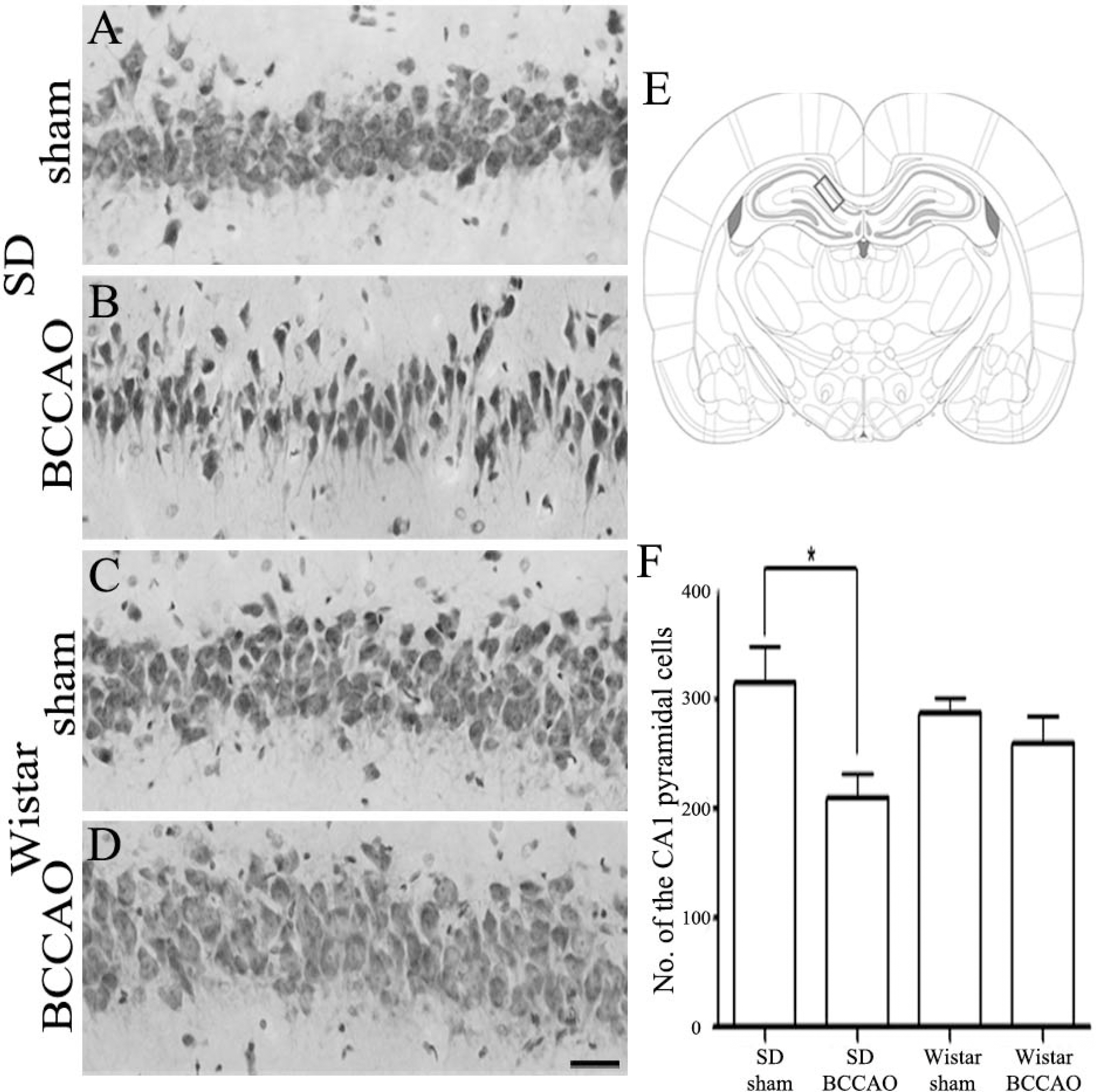
- In order to reproduce chronic cerebral hypoperfusion as it occurs in human aging and Alzheimer's disease, we introduced permanent, bilateral occlusion of the common carotid arteries (BCCAO) in rats (Farkas et al, 2007). Here, we induced BCCAO in two different rat strains in order to determine whether there was a strain difference in the pathogenic response to BCCAO. Male Wistar and Sprague-Dawley (SD) rats (250-270 g) were subjected to BCCAO for three weeks. Kluver-Barrera and cresyl violet staining were used to evaluate white matter and gray matter damage, respectively. Wistar rats had a considerably higher mortality rate (four of 14 rats) as compared to SD rats (one of 15 rats) following BCCAO. Complete loss of pupillary light reflex occurred in all Wistar rats that survived, but loss of pupillary light reflex did not occur at all in SD rats. Moreover, BCCAO induced marked vacuolation in the optic tract of Wistar rats as compared to SD rats. In contrast, SD rats showed fewer CA1 hippocampal neurons than Wistar rats following BCCAO. These results suggest that the neuropathological process induced by BCCAO takes place in a region-specific pattern that varies according to the strain of rat involved.
Keyword
MeSH Terms
Figure
Reference
-
Butte DM., Fortin T., Pappas BA. Pinealectomy: behavioral and neuropathological consequences in a chronic cerebral hypoperfusion model. Neurobiol Aging. 23:309–317. 2002.Bennett SA., Tenniswood M., Chen JH., Davidson CM., Keyes MT., Fortin T., Pappas A. Chronic cerebral hypoperfusion elicits neuronal apoptosis and behavioral impairment. Neuroreport. 9:164–166. 1998.
ArticleCho KO., La HO., Cho YJ., Sung KW., Kim SY. Minocycline attenuates white matter damage in a rat model of chronic cerebral hypoperfusion. J Neurosci Res. 83:285–291. 2006.
ArticleDavidson CM., Pappas BA., Stevens WD., Fortin T., Bennett SAL. Chronic cerebral hypoperfusion: loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res. 859:96–103. 2000.
ArticleFarkas E., Institoris A., Domoki F., Mihaly A., Bari F. The effect of pre- and post-treatment with diazoxide on the early phase of chronic cerebral hypoperfusion in the rat. Brain Res. 1087:168–174. 2006.
ArticleFarkas E., Luiten PG., Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 54:162–180. 2007.
ArticleHirabayashi H., Kurita D., Takizawa S., Shinohara Y. Phosphate-related energy compounds are not exhausted in chronically hypoperfused rat brain cortex after cortical spreading depression. J Stroke Cerebrovasc Dis. 3:271–279. 2004.
ArticleJellinger KA. The enigma of vascular cognitive disorder and vascular dementia. Acta. Neuropathol. 113:349–388. 2007.Kuang X., Du JR., Liu YX., Zhang GY., Peng HY. Postischemic administration of Z-Ligustilide ameliorates cognitive dysfunction and brain damage induced by permanent forebrain ischemia in rats. Pharmacol Biochem Behav. 88:213–221. 2008.
ArticleLavinsky D., Arterni NS., Achaval M., Netto CA. Chronic bilateral common carotid artery occlusion: a model for ocular ischemic syndrome in the rat. Graefes Arch Clin Exp Ophthalmol. 244:199–204. 2006.
ArticleLiu C., Wu J., Gu J., Xiong Z., Wang F., Wang J., Wang W., Chen J. Baicalein improves cognitive deficits induced by chronic cerebral hypoperfusion in rats. Pharmacol Biochem Behav. 86:423–430. 2007.
ArticleLiu HX., Zhang JJ., Zheng P., Zhang Y. Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res. 139:169–177. 2005.
ArticleNakaji K., Ihara M., Takahashi C., Itohara S., Noda M., Takahashi R., Tomomoto H. Matrix metalloproteinase-2 plays a critical role in the pathogenesis of white matter lesions after chronic cerebral hypoperfusion in rodents. Stroke. 37:2816–2823. 2006.
ArticleNi JW., Matsumoto K., Li HB., Murakami Y., Watanabe H. Neuronal damage and decrease of centeral acetylcholine level following permanent occlusion of bilateral common carotid arteries in rat. Brain Res. 673:290–296. 1995.Ni JW., Ohta H., Matsumoto K., Watanabe H. Progressive cognitive impairment following chronic cerebral hypoperfusion induced by permanent occlusion of bilateral carotid arteries in rats. Brain Res. 653:231–236. 1994.
ArticleOhta H., Nishikawa H., Kimura H., Anayama H., Miyamoto M. Chronic cerebral hypoperfusion by permanent internal carotid ligation produces learning impairment without brain damage in rat. Neuroscience. 79:1039–1050. 1997.Pantoni L., Garcia JH., Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 27:1641–1646. 1996.
ArticlePappas BA., Torre JC., Davidson CM., Keyes MT., Fortin T. Chronic reduction of cerebral blood flow in the adult rat: late-emerging CA1 cell loss and memory dysfunction. Brain Res. 708:50–58. 1996.
ArticlePaxinos G., Watson C. The rat brain in stereotaxic coordinates. 6th ed.Academic Press;Burlington: 2007.Rockwood K., Wentzel C., Hachinski V., Hogan DB., MacKnight C., McDowell I. Prevalence and outcomes of vascular cognitive impairment. Neurology. 54:447–451. 2000.
ArticleRomán GC., Erkinjuntti T., Wallin A., Pantoni L., Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 1:426–436. 2002.
ArticleSarti C., Pantoni L., Bartolini L., Inzitari D. Cognitive impairment and chronic cerebral hypoperfusion: what can be learned from experimental models. J Neurol Sci. 203-204:263–266. 2002.
ArticleSchmidt-Kastner R., Aguirri-Chen C., Saul I., Yick L., Hamasaki D., Busto R., Ginsberg MD. Astrocytes react to oligemia in the fore-brain induced by chronic bilateral common carotid artery occlusion in rat. Brain Res. 1052:28–39. 2005.Sopala M., Danysz W. Chronic cerebral hypoperfusion in the rat enhances age-related deficits in spatial memory. J Neural Transm. 108:1445–1456. 2001.
ArticleStevens WD., Fortin T., Pappas BA. Retinal and optic nerve degeneration after chronic carotid ligation: time course and role of light exposure. Stroke. 33:1107–1112. 2002.Tsuchiya M., Sako K., Yura S., Yonemasu Y. Local cerebral glucose utilization following acute and chronic bilateral carotid artery ligation in Wistar rat: relation to change in local cerebral blood flow. Exp Brain Res. 95:1–7. 1993.
ArticleWakita H., Tomimoto H., Akiguchi I., Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol. 87:484–492. 1994.
ArticleWakita H., Tomimoto H., Akiguchi I., Kimura J. Dose-dependent, protective effect of FK506 against white matter changes in the rat brain after chronic cerebral ischemia. Brain Res. 792:105–113. 1998.
ArticleWakita H., Tomimoto H., Akiguchi I., Matsuo A., Lin JX., Ihara M., McGeer PL. Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res. 924:63–70. 2002.
ArticleWatanabe T., Zhang N., Liu M., Tanaka R., Mizuno Y., Urabe T. Cilostazol protects against brain white matter damage and cognitive impairment in a rat model of chronic cerebral hypoperfusion. Stroke. 37:1539–1545. 2006.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chronic cerebral hypoperfusion and plasticity of the posterior cerebral artery following permanent bilateral common carotid artery occlusion
- Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia
- Neuroprotective Effect of Duloxetine on Chronic Cerebral Hypoperfusion-Induced Hippocampal Neuronal Damage
- Protection of the brain through supplementation with larch arabinogalactan in a rat model of vascular dementia
- Anesthetic management of a patient with bilateral common carotid and subclavian arteries occlusion using cerebral oximetry monitoring: A case report