Healthc Inform Res.
2011 Dec;17(4):267-275. 10.4258/hir.2011.17.4.267.
The Development of a Korean Drug Dosing Database
- Affiliations
-
- 1Department of Pharmacy, Ewha Womans University Mokdong Hospital, Seoul, Korea.
- 2BIT Computer Co. Ltd., Seoul, Korea.
- 3College of Pharmacy, Ewha Womans University, Seoul, Korea. bklee@ewha.ac.kr
- 4Department of Pharmacy, Seoul National University Bundang Hospital, Seongnam, Korea.
- 5College of Pharmacy, Ajou University, Suwon, Korea.
- 6Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 7Korea Food & Drug Administration, Cheongwon, Korea.
- KMID: 2284554
- DOI: http://doi.org/10.4258/hir.2011.17.4.267
Abstract
OBJECTIVES
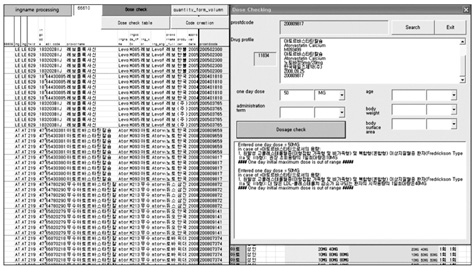
This report describes the development process of a drug dosing database for ethical drugs approved by the Korea Food & Drug Administration (KFDA). The goal of this study was to develop a computerized system that supports physicians' prescribing decisions, particularly in regards to medication dosing.
METHODS
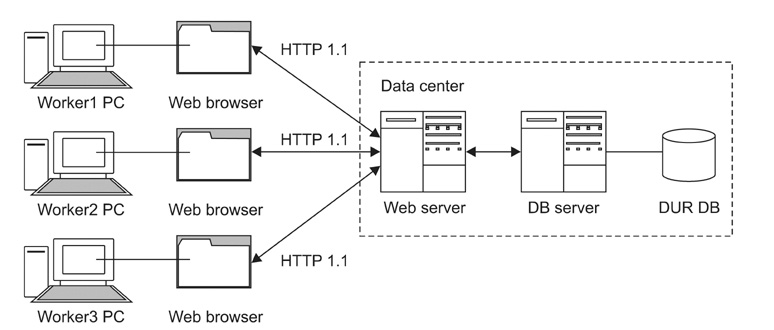
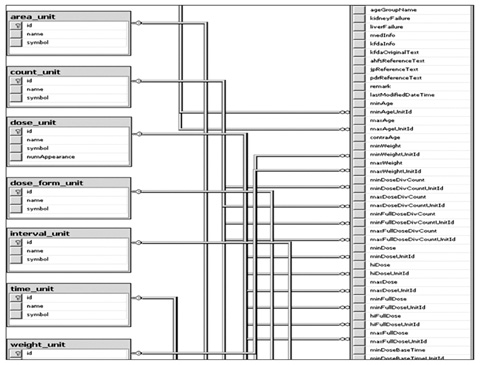
The advisory committee, comprised of doctors, pharmacists, and nurses from the Seoul National University Bundang Hospital, pharmacists familiar with drug databases, KFDA officials, and software developers from the BIT Computer Co. Ltd. analyzed approved KFDA drug dosing information, defined the fields and properties of the information structure, and designed a management program used to enter dosing information. The management program was developed using a web based system that allows multiple researchers to input drug dosing information in an organized manner. The whole process was improved by adding additional input fields and eliminating the unnecessary existing fields used when the dosing information was entered, resulting in an improved field structure.
RESULTS
A total of 16,994 drugs sold in the Korean market in July 2009, excluding the exclusion criteria (e.g., radioactivity drugs, X-ray contrast medium), usage and dosing information were made into a database.
CONCLUSIONS
The drug dosing database was successfully developed and the dosing information for new drugs can be continually maintained through the management mode. This database will be used to develop the drug utilization review standards and to provide appropriate dosing information.
MeSH Terms
Figure
Reference
-
1. Rich D, Menke J, Fisher D. Dose range checking in a computer order entry system. AMIA Annu Symp Proc. 2003. 985.2. Kuperman GJ, Bobb A, Payne TH, Avery AJ, Gandhi TK, Burns G, Classen DC, Bates DW. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007. 14:29–40.
Article3. The U.S. Pharmacopeia Drug Utilization Review Advisory Panel. Drug utilization review: mechanisms to improve its effectiveness and broaden its scope. J Am Pharm Assoc (Wash). 2000. 40:538–545.4. Teich JM, Merchia PR, Schmiz JL, Kuperman GJ, Spurr CD, Bates DW. Effects of computerized physician order entry on prescribing practices. Arch Intern Med. 2000. 160:2741–2747.
Article5. White A. Measuring the benefits of Ajax [Internet]. Inter.com. c2011. cited at 2011 Apr 1. Available from: http://www.developer.com/xml/article.php/3554271.6. Gravano L, Ipeirotis PG, Koudas N, Srivastava D. Text joins in an RDBMS for web data integration. WWW '03 Proceedings of the 12th International Conference on World Wide Web. 2003. New York, NY: Association for Computing Machinery;90–101.
Article7. Grossman DA, Frieder O, Holmes DO, Roberts DC. Integrating structured data and text: a relational approach. J Am Soc Inf Sci. 1997. 48:122–132.
Article8. Seo IY. A survey on the status of computerized provider order entry system's medication dosing. In : The 9th ACCP conference; 2009 Sep 26-28; Seoul, Korea. –195.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of two dosing regimens of aminoglycosides for the development of nephrotoxicity: once versus multiple daily dosing
- Pharmacodynamic principles and target concentration intervention
- Development of Renal Dosing System at a University Hospital
- Development of Database for Clinical Transplantation Research
- Comparative Analysis of Treatment Outcomes Following Regular vs. Irregular Administration of Biologics in Patients with Psoriasis