Ewha Med J.
2012 Sep;35(2):129-134. 10.12771/emj.2012.35.2.129.
Phantom Ischemia Mimicking ST Segment Elevation Myocardial Infarction in Fulminant Myocarditis
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea. xkyhx@hanmail.net
- KMID: 2284005
- DOI: http://doi.org/10.12771/emj.2012.35.2.129
Abstract
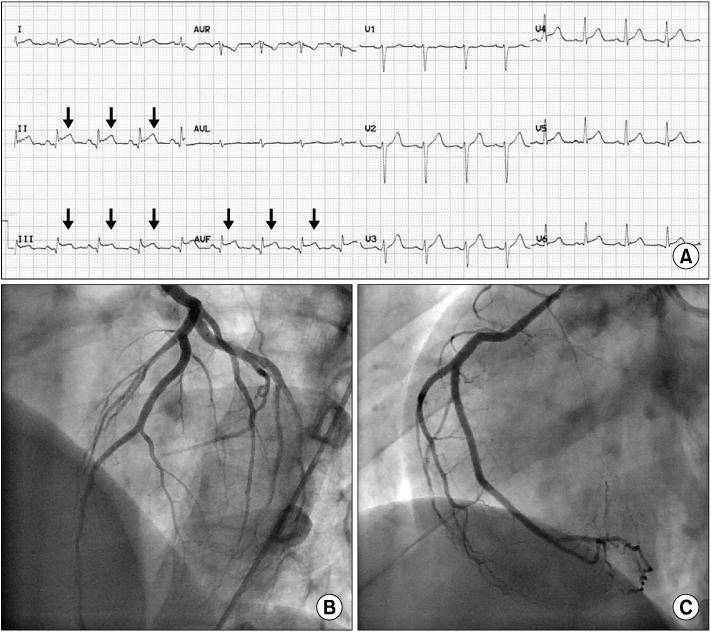
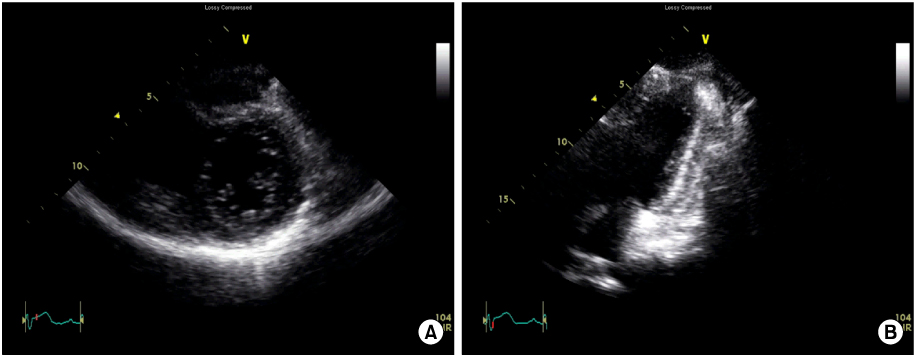
- A 30-year-old man visited the emergency room for chest pain, dyspnea and fever. Despite increased serum cardiac enzymes, ST segment elevation and inferior wall akinesis in electrocardiography and echocardiography, no atherosclerosis was evident in the coronary angiography. However, radionuclide myocardial perfusion image at day 2 showed a persistent perfusion defect in the left ventricular (LV) inferior wall. At day 3, prominent myocardial edema and severe LV systolic dysfunction developed with signs of heart failure. In this case, fulminant myocarditis seemed to originate from the right coronary artery territory and simulated a ST segment elevation myocardial infarction without coronary artery obstruction. The pathogenesis of the localized perfusion defect was unlcear.
MeSH Terms
Figure
Reference
-
1. Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006. 113:876–890.2. Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001. 104:1076–1082.3. Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, et al. Update on myocarditis. J Am Coll Cardiol. 2012. 59:779–792.4. Pankuweit S, Lamparter S, Schoppet M, Maisch B. Parvovirus B19 genome in endomyocardial biopsy specimen. Circulation. 2004. 109:e179.5. McCully RB, Cooper LT, Schreiter S. Coronary artery spasm in lymphocytic myocarditis: a rare cause of acute myocardial infarction. Heart. 2005. 91:202.6. Silva D, Marques P, Martins S, Bordalo E Sa AL, Nobrega J, Duarte J, et al. Coronary artery vasospasm and acute myocarditis: a rare association. Rev Port Cardiol. 2010. 29:1879–1888.7. Shimokawa H. Cellular and molecular mechanisms of coronary artery spasm: lessons from animal models. Jpn Circ J. 2000. 64:1–12.8. Gibelli G, Devizzi S, Brioschi P, Rimini A, Biasi S. Sudden ST elevation with angina-like pain in myocarditis. An uncommon course of a common disease: strategic role of cardiac magnetic resonance. J Cardiovasc Med (Hagerstown). 2009. 10:264–266.9. Yilmaz A, Mahrholdt H, Athanasiadis A, Vogelsberg H, Meinhardt G, Voehringer M, et al. Coronary vasospasm as the underlying cause for chest pain in patients with PVB19 myocarditis. Heart. 2008. 94:1456–1463.10. Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004. 109:1250–1258.11. Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006. 114:1581–1590.12. Kandolf R. Virus etiology of inflammatory cardiomyopathy. Dtsch Med Wochenschr. 2004. 129:2187–2192.13. Bultmann BD, Klingel K, Sotlar K, Bock CT, Baba HA, Sauter M, et al. Fatal parvovirus B19-associated myocarditis clinically mimicking ischemic heart disease: an endothelial cell-mediated disease. Hum Pathol. 2003. 34:92–95.14. Prober C. Sixth disease and the ubiquity of human herpesviruses. N Engl J Med. 2005. 352:753–755.15. Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007. 50:1914–1931.16. Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss HP, McCarthy R, et al. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003. 42:466–472.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Acute Myopericarditis with Localized ST Elevation Mimicking Myocardial Infarction
- A Case of Acute Fulminant Myocarditis Progressed into and Recovered from Congestive Heart Failure and Multiorgan Failure
- The Eletrocardiographic Analysis of Acute Myocardial Infarction and Non-infarction Syndrome In the Patients with ST Segment Elevation and Chest Pain
- A Case of ST-Segment Elevation in a Patient with Subarachnoid Hemorrhage
- ST segment