Diabetes Metab J.
2011 Aug;35(4):309-316. 10.4093/dmj.2011.35.4.309.
Glycosphingolipid Modification: Structural Diversity, Functional and Mechanistic Integration of Diabetes
- Affiliations
-
- 1Graduate School of Advanced Life Science, Hokkaido University, Sapporo, Japan. ty11106@sci.hokudai.ac.jp
- 2World Class University Program, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2281636
- DOI: http://doi.org/10.4093/dmj.2011.35.4.309
Abstract
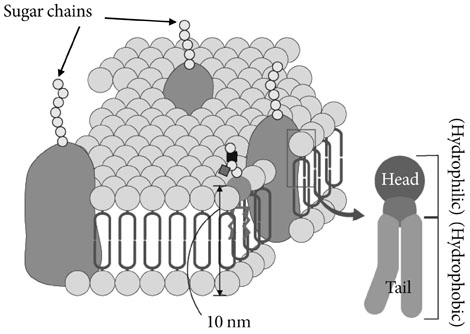
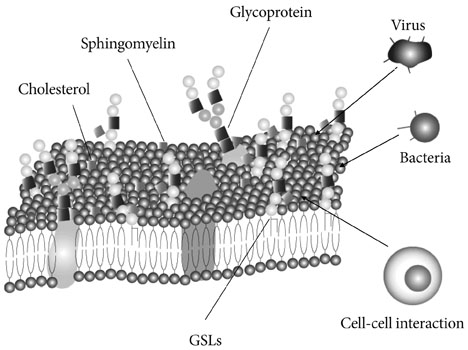
- Glycosphingolipids (GSLs) are present in all mammalian cell plasma membranes and intracellular membrane structures. They are especially concentrated in plasma membrane lipid domains that are specialized for cell signaling. Plasma membranes have typical structures called rafts and caveola domain structures, with large amounts of sphingolipids, cholesterol, and sphingomyelin. GSLs are usually observed in many organs ubiquitously. However, GSLs, including over 400 derivatives, participate in diverse cellular functions. Several studies indicate that GSLs might have an effect on signal transduction related to insulin receptors and epidermal growth factor receptors. GSLs may modulate immune responses by transmitting signals from the exterior to the interior of the cell. Guillain-Barre syndrome is one of the autoimmune disorders characterized by symmetrical weakness in the muscles of the legs. The targets of the immune response are thought to be gangliosides, which are one group of GSLs. Other GSLs may serve as second messengers in several signaling pathways that are important to cell survival or programmed cell death. In the search for clear evidence that GSLs may play critical roles in various biological functions, many researchers have made genetically engineered mice. Before the era of gene manipulation, spontaneous animal models or chemical-induced disease models were used.
MeSH Terms
-
Animals
Caveolae
Cell Death
Cell Membrane
Cell Survival
Cholesterol
Diabetes Mellitus
Gangliosides
Glycosphingolipids
Guillain-Barre Syndrome
Intracellular Membranes
Leg
Mice
Models, Animal
Muscles
Receptor, Epidermal Growth Factor
Receptor, Insulin
Second Messenger Systems
Signal Transduction
Sphingolipids
Cholesterol
Gangliosides
Glycosphingolipids
Receptor, Epidermal Growth Factor
Receptor, Insulin
Sphingolipids
Figure
Reference
-
1. Kolter T, Sandhoff K. Recent advances in the biochemistry of sphingolipidoses. Brain Pathol. 1998. 8:79–100.2. Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990. 265:18713–18716.3. Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010. 688:1–23.4. Kasahara K, Sanai Y. Functional roles of glycosphingolipids in signal transduction via lipid rafts. Glycoconj J. 2000. 17:153–162.5. Korade Z, Kenworthy AK. Lipid rafts, cholesterol, and the brain. Neuropharmacology. 2008. 55:1265–1273.6. Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009. 50:Suppl. S323–S328.7. Rietveld A, Simons K. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim Biophys Acta. 1998. 1376:467–479.8. Saeki K, Fukuyama S, Ayada T, Nakaya M, Aki D, Takaesu G, Hanada T, Matsumura Y, Kobayashi T, Nakagawa R, Yoshimura A. A major lipid raft protein raftlin modulates T cell receptor signaling and enhances th17-mediated autoimmune responses. J Immunol. 2009. 182:5929–5937.9. Sohn HW, Pierce SK, Tzeng SJ. Live cell imaging reveals that the inhibitory FcgammaRIIB destabilizes B cell receptor membrane-lipid interactions and blocks immune synapse formation. J Immunol. 2008. 180:793–799.10. Kinoshita MO, Furuya S, Ito S, Shinoda Y, Yamazaki Y, Greimel P, Ito Y, Hashikawa T, Machida T, Nagatsuka Y, Hirabayashi Y. Lipid rafts enriched in phosphatidylglucoside direct astroglial differentiation by regulating tyrosine kinase activity of epidermal growth factor receptors. Biochem J. 2009. 419:565–575.11. Sanchez-Wandelmer J, Davalos A, de la Pena G, Cano S, Giera M, Canfran-Duque A, Bracher F, Martin-Hidalgo A, Fernandez-Hernando C, Lasuncion MA, Busto R. Haloperidol disrupts lipid rafts and impairs insulin signaling in SH-SY5Y cells. Neuroscience. 2010. 167:143–153.12. Kim KB, Kim BW, Choo HJ, Kwon YC, Ahn BY, Choi JS, Lee JS, Ko YG. Proteome analysis of adipocyte lipid rafts reveals that gC1qR plays essential roles in adipogenesis and insulin signal transduction. Proteomics. 2009. 9:2373–2382.13. Yamashita T, Wada R, Sasaki T, Deng C, Bierfreund U, Sandhoff K, Proia RL. A vital role for glycosphingolipid synthesis during development and differentiation. Proc Natl Acad Sci U S A. 1999. 96:9142–9147.14. Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol. 2004. 6:393–404.15. Hakomori S, Igarashi Y. Functional role of glycosphingolipids in cell recognition and signaling. J Biochem. 1995. 118:1091–1103.16. Nagai Y, Tsuji S. Significance of ganglioside-mediated glycosignal transduction in neuronal differentiation and development. Prog Brain Res. 1994. 101:119–126.17. Ichikawa S, Hirabayashi Y. Glucosylceramide synthase and glycosphingolipid synthesis. Trends Cell Biol. 1998. 8:198–202.18. Schnaar RL. Glycosphingolipids in cell surface recognition. Glycobiology. 1991. 1:477–485.19. Tettamanti G, Riboni L. Gangliosides and modulation of the function of neural cells. Adv Lipid Res. 1993. 25:235–267.20. Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997. 387:569–572.21. Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997. 9:534–542.22. Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998. 67:199–225.23. Whitmore CD, Hindsgaul O, Palcic MM, Schnaar RL, Dovichi NJ. Metabolic cytometry. Glycosphingolipid metabolism in single cells. Anal Chem. 2007. 79:5139–5142.24. Memon RA, Holleran WM, Uchida Y, Moser AH, Ichikawa S, Hirabayashi Y, Grunfeld C, Feingold KR. Regulation of glycosphingolipid metabolism in liver during the acute phase response. J Biol Chem. 1999. 274:19707–19713.25. Tettamanti G. Ganglioside/glycosphingolipid turnover: new concepts. Glycoconj J. 2004. 20:301–317.26. White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994. 269:1–4.27. Hubbard SR, Wei L, Ellis L, Hendrickson WA. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 1994. 372:746–754.28. Schinner S, Scherbaum WA, Bornstein SR, Barthel A. Molecular mechanisms of insulin resistance. Diabet Med. 2005. 22:674–682.29. Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007. 9:316–323.30. Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000. 1:31–39.31. Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008. 9:139–150.32. Vainio S, Heino S, Mansson JE, Fredman P, Kuismanen E, Vaarala O, Ikonen E. Dynamic association of human insulin receptor with lipid rafts in cells lacking caveolae. EMBO Rep. 2002. 3:95–100.33. Ottenhoff R, Powlson AS, Grefhorst A, van Eijk M, Dubbelhuis PF, Aten J, Kuipers F, Serlie MJ, Wennekes T, Sethi JK, O'Rahilly S, Overkleeft HS. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007. 56:1341–1349.34. Radin NS, Shayman JA, Inokuchi J. Metabolic effects of inhibiting glucosylceramide synthesis with PDMP and other substances. Adv Lipid Res. 1993. 26:183–213.35. Kobayashi T, Takahashi M, Nagatsuka Y, Hirabayashi Y. Lipid rafts: new tools and a new component. Biol Pharm Bull. 2006. 29:1526–1531.36. Pagliassotti MJ, Prach PA, Koppenhafer TA, Pan DA. Changes in insulin action, triglycerides, and lipid composition during sucrose feeding in rats. Am J Physiol. 1996. 271(5 Pt 2):R1319–R1326.37. Chicco A, D'Alessandro ME, Karabatas L, Pastorale C, Basabe JC, Lombardo YB. Muscle lipid metabolism and insulin secretion are altered in insulin-resistant rats fed a high sucrose diet. J Nutr. 2003. 133:127–133.38. Robbez Masson V, Lucas A, Gueugneau AM, Macaire JP, Paul JL, Grynberg A, Rousseau D. Long-chain (n-3) polyunsaturated fatty acids prevent metabolic and vascular disorders in fructose-fed rats. J Nutr. 2008. 138:1915–1922.39. Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999. 48:927–932.40. Johnson PR, Zucker LM, Cruce JA, Hirsch J. Cellularity of adipose depots in the genetically obese Zucker rat. J Lipid Res. 1971. 12:706–714.41. Schonfeld G, Felski C, Howald MA. Characterization of the plasma lipoproteins of the genetically obese hyperlipoproteinemic Zucker fatty rat. J Lipid Res. 1974. 15:457–464.42. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992. 41:1422–1428.43. Shima K, Shi K, Sano T, Iwami T, Mizuno A, Noma Y. Is exercise training effective in preventing diabetes mellitus in the Otsuka-Long-Evans-Tokushima fatty rat, a model of spontaneous non-insulin-dependent diabetes mellitus? Metabolism. 1993. 42:971–977.44. Nagata R, Nishio Y, Sekine O, Nagai Y, Maeno Y, Ugi S, Maegawa H, Kashiwagi A. Single nucleotide polymorphism (-468 Gly to A) at the promoter region of SREBP-1c associates with genetic defect of fructose-induced hepatic lipogenesis [corrected]. J Biol Chem. 2004. 279:29031–29042.45. Sumiyoshi M, Sakanaka M, Kimura Y. Chronic intake of high-fat and high-sucrose diets differentially affects glucose intolerance in mice. J Nutr. 2006. 136:582–587.46. Farah V, Elased KM, Chen Y, Key MP, Cunha TS, Irigoyen MC, Morris M. Nocturnal hypertension in mice consuming a high fructose diet. Auton Neurosci. 2006. 130:41–50.47. Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995. 44:645–651.48. Nakamura M. A diabetic strain of the mouse. Proc Jpn Acad Ser B Phys Biol Sci. 1962. 38:348–352.49. Reddi AS, Camerini-Davalos RA. Hereditary diabetes in the KK mouse: an overview. Adv Exp Med Biol. 1988. 246:7–15.50. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998. 395:763–770.51. Sima AA, Robertson DM. Peripheral neuropathy in mutant diabetic mouse [C57BL/Ks (db/db)]. Acta Neuropathol. 1978. 41:85–89.52. Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 2000. 49:22–31.53. Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992. 51:285–322.54. Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008. 455:1109–1113.55. Chatzigeorgiou A, Halapas A, Kalafatakis K, Kamper E. The use of animal models in the study of diabetes mellitus. In Vivo. 2009. 23:245–258.56. Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008. 51:216–226.57. Sato C, Shikata K, Hirota D, Sasaki M, Nishishita S, Miyamoto S, Kodera R, Ogawa D, Tone A, Kataoka HU, Wada J, Kajitani N, Makino H. P-selectin glycoprotein ligand-1 deficiency is protective against obesity-related insulin resistance. Diabetes. 2011. 60:189–199.58. Inoue T, Tsuzuki Y, Matsuzaki K, Matsunaga H, Miyazaki J, Hokari R, Okada Y, Kawaguchi A, Nagao S, Itoh K, Matsumoto S, Miura S. Blockade of PSGL-1 attenuates CD14+ monocytic cell recruitment in intestinal mucosa and ameliorates ileitis in SAMP1/Yit mice. J Leukoc Biol. 2005. 77:287–295.59. Rivera-Nieves J, Burcin TL, Olson TS, Morris MA, McDuffie M, Cominelli F, Ley K. Critical role of endothelial P-selectin glycoprotein ligand 1 in chronic murine ileitis. J Exp Med. 2006. 203:907–917.60. Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, Werth N, Sandhoff R, Sandhoff K, Proia RL. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci U S A. 2003. 100:3445–3449.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Importance of Lifestyle in the Prevention and Trearment of Diabetes
- Structural Equation Modeling on Life-world Integration in People with Severe Burns
- Response: Genome-Wide Association Study Identifies Two Novel Loci with Sex-Specific Effects for Type 2 Diabetes Mellitus and Glycemic Traits in a Korean Population (Diabetes Metab J 2014;38:375-87)
- Diabetes and Periodontal Disease
- Functional and Mechanistic Integration of Infection and the Metabolic Syndrome