Diabetes Metab J.
2012 Apr;36(2):98-107. 10.4093/dmj.2012.36.2.98.
The Roles of Glycated Albumin as Intermediate Glycation Index and Pathogenic Protein
- Affiliations
-
- 1Severance Executive Healthcare Clinic, Yonsei University Health System, Seoul, Korea.
- 2Division of Endocrinology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. bwanlee@yuhs.ac
- KMID: 2281411
- DOI: http://doi.org/10.4093/dmj.2012.36.2.98
Abstract
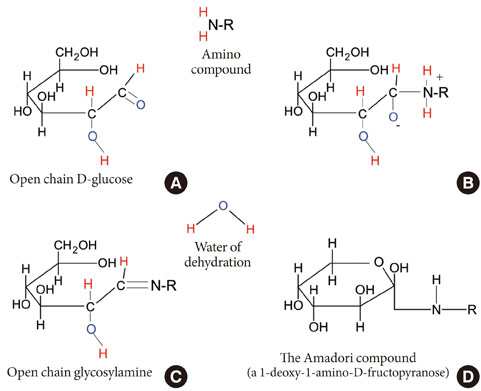
- The conventional glycemic indices used in management of diabetic patients includes A1c, fructosamine, 1,5-anhydroglucitol, and glycated albumin (GA). Among these indices, A1c is currently used as the gold standard. However, A1c cannot reflect the glycemic change over a relatively short period of time, and its accuracy is known to decrease when abnormalities in hemoglobin metabolism, such as anemia, coexist. When considering these weaknesses, there have been needs for finding a novel glycemic index for diagnosing and managing diabetes, as well as for predicting diabetic complications properly. Recently, several studies have suggested the potential of GA as an intermediate-term glycation index in covering the short-term effect of treatment. Furthermore, its role as a pathogenic protein affecting the worsening of diabetes and occurrence of diabetic complications is receiving attention as well. Therefore, in this article, we wanted to review the recent status of GA as a glycemic index and as a pathogenic protein.
MeSH Terms
Figure
Cited by 1 articles
-
Hemoglobin A1c, Not Glycated Albumin, Can Independently Reflect the Ankylosing Spondylitis Disease Activity Score
Sejin Byun, Seung Min Jung, Jason Jungsik Song, Yong-Beom Park, Sang-Won Lee
J Rheum Dis. 2018;25(2):131-139. doi: 10.4078/jrd.2018.25.2.131.
Reference
-
1. Turner RC, Cull CA, Frighi V, Holman RR. UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999. 281:2005–2012.2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008. 359:1577–1589.3. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012. 35:Suppl 1. S64–S71.5. True MW. Circulating biomarkers of glycemia in diabetes management and implications for personalized medicine. J Diabetes Sci Technol. 2009. 3:743–747.6. Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010. 57:751–762.7. Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987. 33:2153–2163.8. Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001. 56:1–21.9. Lyons TJ, Baynes JW, Patrick JS, Colwell JA, Lopes-Virella MF. Glycosylation of low density lipoprotein in patients with type 1 (insulin-dependent) diabetes: correlations with other parameters of glycaemic control. Diabetologia. 1986. 29:685–689.10. Rondeau P, Bourdon E. The glycation of albumin: structural and functional impacts. Biochimie. 2011. 93:645–658.11. Buse JB, Freeman JL, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark ): a short-term glycemic marker. Diabetes Technol Ther. 2003. 5:355–363.12. Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, Wittlin S. 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006. 29:1214–1219.13. Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability: does it matter? Endocr Rev. 2010. 31:171–182.14. Kim MJ, Jung HS, Hwang-Bo Y, Cho SW, Jang HC, Kim SY, Park KS. Evaluation of 1,5-anhydroglucitol as a marker for glycemic variability in patients with type 2 diabetes mellitus. Acta Diabetol. Epub 2011 Jun 18. DOI: 10.1007/s00592-011-0302-0.15. Cohen MP, Clements RS. Measuring glycated proteins: clinical and methodological aspects. Diabetes Technol Ther. 1999. 1:57–70.16. Stettler C, Stahl M, Allemann S, Diem P, Schmidlin K, Zwahlen M, Riesen W, Keller U, Christ E. Association of 1,5-anhydroglucitol and 2-h postprandial blood glucose in type 2 diabetic patients. Diabetes Care. 2008. 31:1534–1535.17. Suwa T, Ohta A, Matsui T, Koganei R, Kato H, Kawata T, Sada Y, Ishii S, Kondo A, Murakami K, Katabami T, Tanaka Y. Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM). Endocr J. 2010. 57:135–140.18. Evans TW. Review article: albumin as a drug: biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther. 2002. 16:Suppl 5. 6–11.19. Prajapati KD, Sharma SS, Roy N. Current perspectives on potential role of albumin in neuroprotection. Rev Neurosci. 2011. 22:355–363.20. Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008. 582:1783–1787.21. Bourdon E, Blache D. The importance of proteins in defense against oxidation. Antioxid Redox Signal. 2001. 3:293–311.22. Halliwell B. Albumin: an important extracellular antioxidant? Biochem Pharmacol. 1988. 37:569–571.23. Carballal S, Alvarez B, Turell L, Botti H, Freeman BA, Radi R. Sulfenic acid in human serum albumin. Amino Acids. 2007. 32:543–551.24. Kragh-Hansen U, Chuang VT, Otagiri M. Practical aspects of the ligand-binding and enzymatic properties of human serum albumin. Biol Pharm Bull. 2002. 25:695–704.25. Levine RL, Berlett BS, Moskovitz J, Mosoni L, Stadtman ER. Methionine residues may protect proteins from critical oxidative damage. Mech Ageing Dev. 1999. 107:323–332.26. Ahmed N, Furth AJ. Failure of common glycation assays to detect glycation by fructose. Clin Chem. 1992. 38:1301–1303.27. McPherson JD, Shilton BH, Walton DJ. Role of fructose in glycation and cross-linking of proteins. Biochemistry. 1988. 27:1901–1907.28. Hogan M, Cerami A, Bucala R. Advanced glycosylation endproducts block the antiproliferative effect of nitric oxide: role in the vascular and renal complications of diabetes mellitus. J Clin Invest. 1992. 90:1110–1115.29. Mullarkey CJ, Edelstein D, Brownlee M. Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun. 1990. 173:932–939.30. Rubenstein DA, Maria Z, Yin W. Glycated albumin modulates endothelial cell thrombogenic and inflammatory responses. J Diabetes Sci Technol. 2011. 5:703–713.31. Cohen MP, Shea E, Chen S, Shearman CW. Glycated albumin increases oxidative stress, activates NF-kappa B and extracellular signal-regulated kinase (ERK), and stimulates ERK-dependent transforming growth factor-beta 1 production in macrophage RAW cells. J Lab Clin Med. 2003. 141:242–249.32. Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med. 2011. 154:303–309.33. Lee EY, Lee BW, Kim D, Lee YH, Kim KJ, Kang ES, Cha BS, Lee EJ, Lee HC. Glycated albumin is a useful glycation index for monitoring fluctuating and poorly controlled type 2 diabetic patients. Acta Diabetol. 2011. 48:167–172.34. Won HK, Kim KJ, Lee BW, Kang ES, Cha BS, Lee HC. Reduction in glycated albumin can predict change in HbA1c: comparison of oral hypoglycaemic agent and insulin treatments. Diabet Med. 2012. 29:74–79.35. Lee YH, Lee BW, Chun SW, Cha BS, Lee HC. Predictive characteristics of patients achieving glycaemic control with insulin after sulfonylurea failure. Int J Clin Pract. 2011. 65:1076–1084.36. Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol. 2008. 2:1114–1121.37. Mehrotra R, Kalantar-Zadeh K, Adler S. Assessment of glycemic control in dialysis patients with diabetes: glycosylated hemoglobin or glycated albumin? Clin J Am Soc Nephrol. 2011. 6:1520–1522.38. Tahara Y. Analysis of the method for conversion between levels of HbA1c and glycated albumin by linear regression analysis using a measurement error model. Diabetes Res Clin Pract. 2009. 84:224–229.39. Garlick RL, Mazer JS. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J Biol Chem. 1983. 258:6142–6146.40. Iberg N, Fluckiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J Biol Chem. 1986. 261:13542–13545.41. Koga M, Murai J, Saito H, Kasayama S. Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care. 2010. 33:270–272.42. Furusyo N, Koga T, Ai M, Otokozawa S, Kohzuma T, Ikezaki H, Schaefer EJ, Hayashi J. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia. 2011. 54:3028–3036.43. Takahashi S, Uchino H, Shimizu T, Kanazawa A, Tamura Y, Sakai K, Watada H, Hirose T, Kawamori R, Tanaka Y. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J. 2007. 54:139–144.44. Kim D, Kim KJ, Huh JH, Lee BW, Kang ES, Cha BS, Lee HC. The ratio of glycated albumin to glycated haemoglobin correlates with insulin secretory function. Clin Endocrinol (Oxf). Epub 2011 Dec 9. DOI: 10.1111/j.1365-2265.2011.04312.x.45. Koga M, Murai J, Saito H, Mukai M, Matsumoto S, Kasayama S. Glycated albumin levels are higher relative to glycated haemoglobin levels in gastrectomized subjects. Ann Clin Biochem. 2010. 47(Pt 1):39–43.46. Kim MK, Kwon HS, Baek KH, Lee JH, Park WC, Sohn HS, Lee KW, Song KH. Effects of thyroid hormone on A1C and glycated albumin levels in nondiabetic subjects with overt hypothyroidism. Diabetes Care. 2010. 33:2546–2548.47. Schaefer EJ, Audelin MC, McNamara JR, Shah PK, Tayler T, Daly JA, Augustin JL, Seman LJ, Rubenstein JJ. Comparison of fasting and postprandial plasma lipoproteins in subjects with and without coronary heart disease. Am J Cardiol. 2001. 88:1129–1133.48. Hiramatsu Y, Shimizu I, Omori Y, Nakabayashi M. Determination of reference intervals of glycated albumin and hemoglobin A1c in healthy pregnant Japanese women and analysis of their time courses and influencing factors during pregnancy. Endocr J. 2012. 59:145–151.49. Ma XJ, Pan JM, Bao YQ, Zhou J, Tang JL, Li Q, Xiang KS, Jia WP. Combined assessment of glycated albumin and fasting plasma glucose improves the detection of diabetes in Chinese subjects. Clin Exp Pharmacol Physiol. 2010. 37:974–979.50. Lu L, Pu LJ, Zhang Q, Wang LJ, Kang S, Zhang RY, Chen QJ, Wang JG, De Caterina R, Shen WF. Increased glycated albumin and decreased esRAGE levels are related to angiographic severity and extent of coronary artery disease in patients with type 2 diabetes. Atherosclerosis. 2009. 206:540–545.51. Rodino-Janeiro BK, Gonzalez-Peteiro M, Ucieda-Somoza R, Gonzalez-Juanatey JR, Alvarez E. Glycated albumin, a precursor of advanced glycation end-products, up-regulates NADPH oxidase and enhances oxidative stress in human endothelial cells: molecular correlate of diabetic vasculopathy. Diabetes Metab Res Rev. 2010. 26:550–558.52. Lee BW, Ihm J, Kang JG, Choi MG, Yoo HJ, Ihm SH. Amadori-glycated albumin-induced vascular smooth muscle cell proliferation and expression of inhibitor of apoptosis protein-1 and nerve growth factor-gamma. Biofactors. 2007. 31:145–153.53. Li Y, Wang S. Glycated albumin activates NADPH oxidase in rat mesangial cells through up-regulation of p47phox. Biochem Biophys Res Commun. 2010. 397:5–11.54. Kumeda Y, Inaba M, Shoji S, Ishimura E, Inariba H, Yabe S, Okamura M, Nishizawa Y. Significant correlation of glycated albumin, but not glycated haemoglobin, with arterial stiffening in haemodialysis patients with type 2 diabetes. Clin Endocrinol (Oxf). 2008. 69:556–561.55. Kim HM, Kim KJ, Moon JH, Lee HJ, Chae MK, Chang HJ, Kang ES, Cha BS, Lee HC, Kim YJ, Lee BW. Association between EPCs count and rate of coronary revascularization in asymptomatic type 2 diabetic patients. Acta Diabetol. Epub 2011 Dec 13. DOI: 10.1007/s00592-011-0360-3.56. Bourdon E, Loreau N, Lagrost L, Blache D. Differential effects of cysteine and methionine residues in the antioxidant activity of human serum albumin. Free Radic Res. 2005. 39:15–20.57. Lyons TJ. Glycation and oxidation: a role in the pathogenesis of atherosclerosis. Am J Cardiol. 1993. 71:26B–31B.58. Faure P, Troncy L, Lecomte M, Wiernsperger N, Lagarde M, Ruggiero D, Halimi S. Albumin antioxidant capacity is modified by methylglyoxal. Diabetes Metab. 2005. 31:169–177.59. Chesne S, Rondeau P, Armenta S, Bourdon E. Effects of oxidative modifications induced by the glycation of bovine serum albumin on its structure and on cultured adipose cells. Biochimie. 2006. 88:1467–1477.60. Shiraki T, Miura Y, Sawada T, Okada T, Sakuraoka Y, Muto T, Kubota K. Glycated albumin suppresses glucose-induced insulin secretion by impairing glucose metabolism in rat pancreatic beta-cells. Nutr Metab (Lond). 2011. 8:20.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Consistency of the Glycation Gap with the Hemoglobin Glycation Index Derived from a Continuous Glucose Monitoring System
- Inhibition of advanced glycation end product formation by burdock root extract
- Hemoglobin A1c, Not Glycated Albumin, Can Independently Reflect the Ankylosing Spondylitis Disease Activity Score
- Outer Blood-retinal Barrier Alteration Induced by Intraocular Ad vanced Glycation Endproduct
- FGF2(bFGF) glycation induced by TGF-beta1 in human lens epithelial cells