Korean J Perinatol.
2015 Sep;26(3):167-173. 10.14734/kjp.2015.26.3.167.
Maternal Preeclampsia and Bronchopulmonary Dysplasia
- Affiliations
-
- 1Department of Pediatrics, Dongguk University Ilsan Hospital, Ilsan, Korea. dayeong1@dumc.or.kr
- KMID: 2281219
- DOI: http://doi.org/10.14734/kjp.2015.26.3.167
Abstract
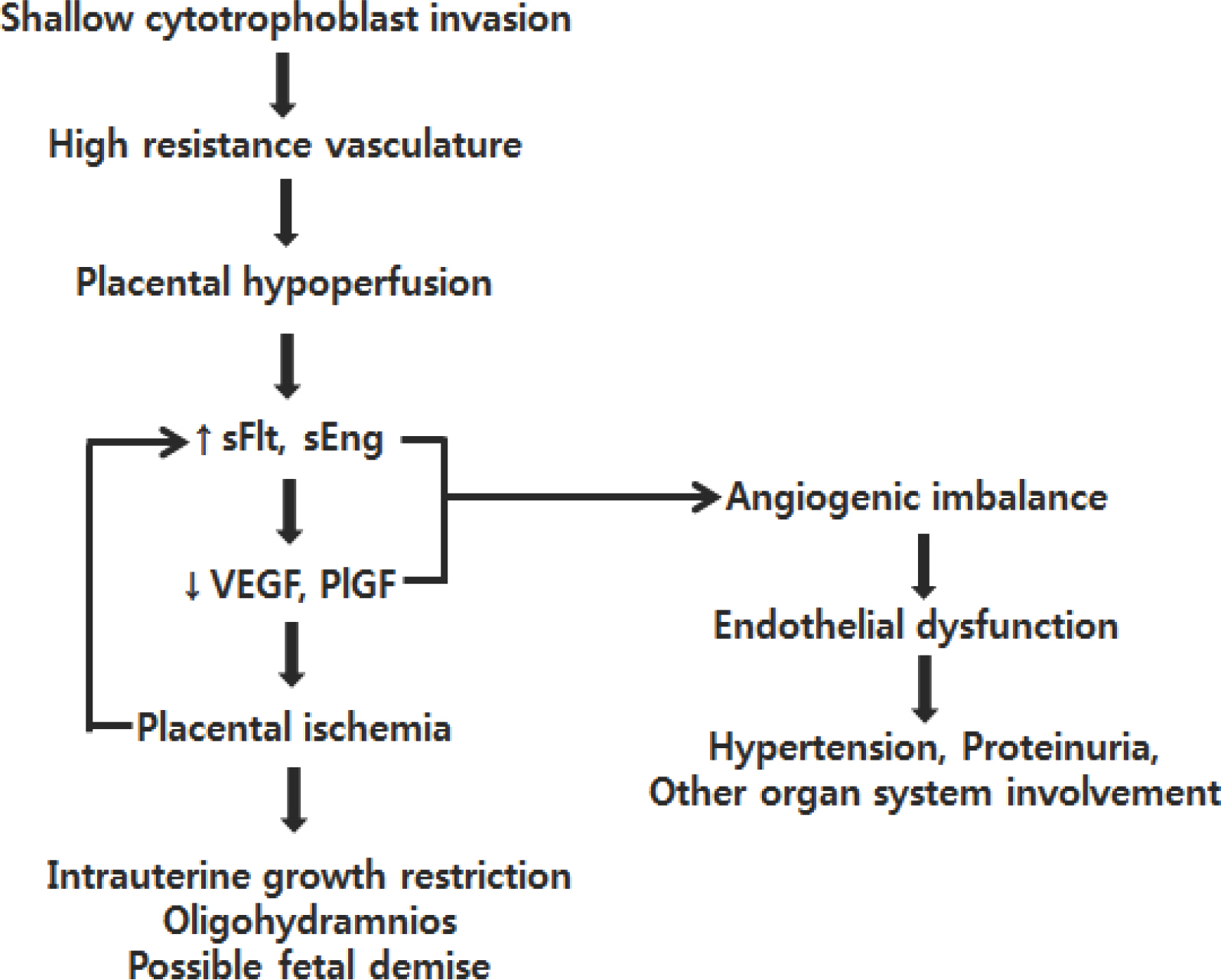
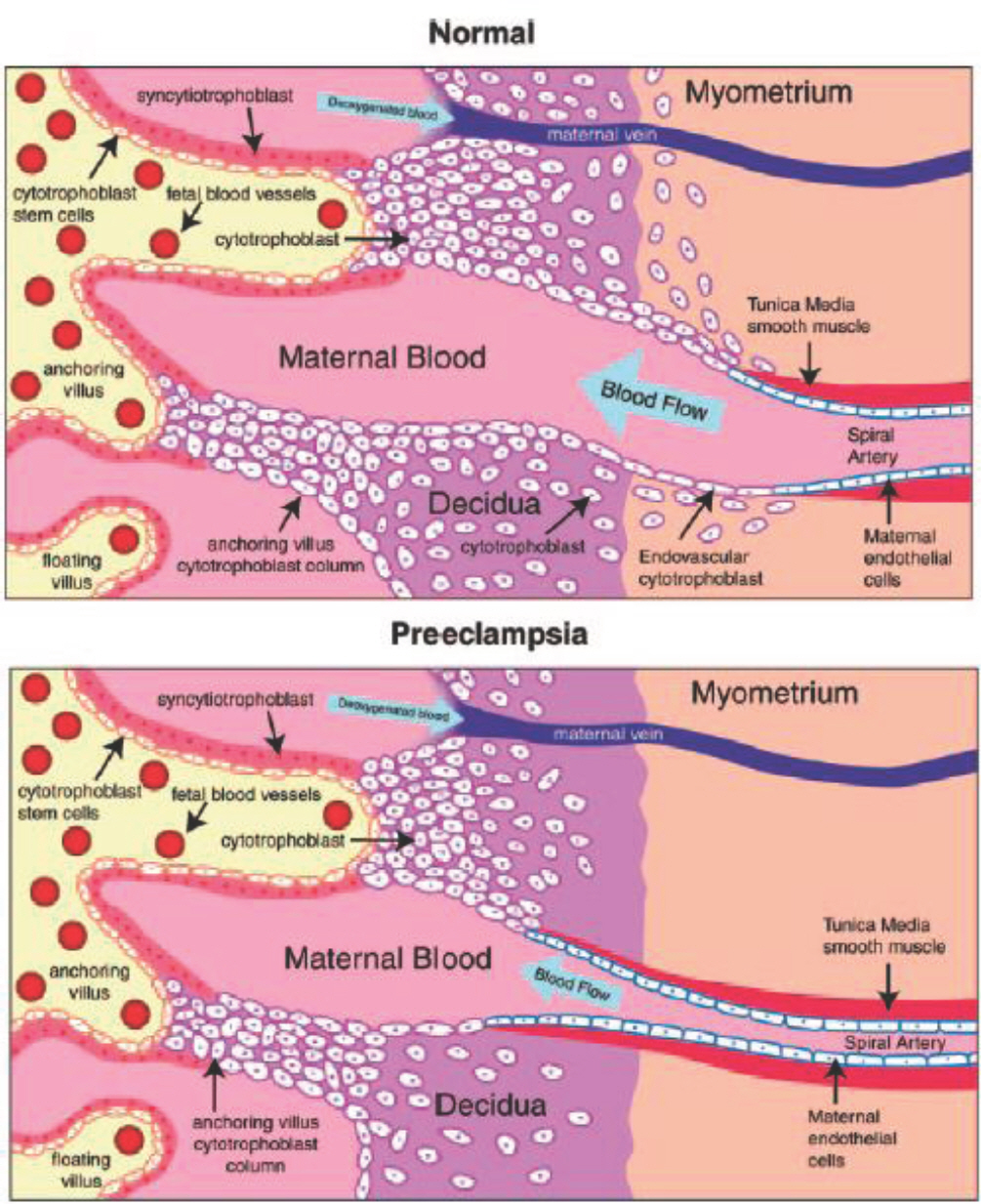
- Preeclampsia is one of the most common complications of pregnancy that is prevalent worldwide, resulting in substantial maternal and neonatal morbidity and mortality. Although the cause remains unclear, preeclampsia may be initiated by abnormal placentation and reduced placental perfusion, followed by an imbalance of angiogenic and antiangiogenic factors and subsequent systemic endothelial dysfunction. High level of antiangiogenic factors, such as soluble vascular endothelial growth factor (VEGF) receptor 1 (sVEGFR-1, also known as sFlt-1) and soluble endoglin, and low levels of angiogenic factors, such as free maternal VEGF and placental growth factor (PlGF), are associated with preeclampsia. Angiogenic and antiangiogenic factors also play an important role during lung angiogenesis, and an imbalance between the two types of factors triggered by inflammation disrupts angiogenesis in bronchopulmonary dysplasia (BPD). Because preeclampsia represents an antiangiogenic state, preterm infants born to mothers with preeclampsia would be at increased risk of developing BPD due to impaired lung development. Recently, preeclampsia has been shown to be independently associated with a high risk for BPD. I have reviewed recent progress in research concerning the correlation between preeclampsia and BPD in aspect of pathophysiology and epidemiology.
MeSH Terms
-
Angiogenesis Inducing Agents
Bronchopulmonary Dysplasia*
Epidemiology
Humans
Infant, Newborn
Infant, Premature
Inflammation
Lung
Mortality
Mothers
Perfusion
Placentation
Pre-Eclampsia*
Pregnancy
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor Receptor-1
Angiogenesis Inducing Agents
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor Receptor-1
Figure
Reference
-
References
1. Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol. 2011; 31:33–46.
Article2. Sibai BM: Publications Committee, Society for Maternal-Fetal Medicine. Evaluation and management of severe preeclampsia before 34 weeks'gestation. Am J Obstet Gynecol. 2011; 205:191–8.3. McDonnold M, Olson G. Preeclampsia: pathophysiology, management, and maternal and fetal sequelae, Neoreviews. 2013; 14:e4–12.4. Basso O, Rasmussen S, Weinberg CR, Wilcox AJ, Irgens LM, Skjaerven R. Trends in fetal and infant survival following preeclampsia. JAMA. 2006; 296:1357–62.
Article5. McElrath TF, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008; 168:980–9.
Article6. Bose C, Van Marter LJ, Laughon M, O'Shea TM, Allred EN, Karna P, et al. Extremely Low Gestational Age Newborn Study Investigators. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics. 2009; 124:e450–8.7. Hansen AR, Barnés CM, Folkman J, McElrath TF. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr. 2010; 156:532–6.
Article8. Ozkan H, Cetinkaya M, Koksal N. Increased incidence of bronchopulmonary dysplasia in preterm infants exposed to preeclampsia. J Matern Fetal Neonatal Med. 2012; 25:2681–5.
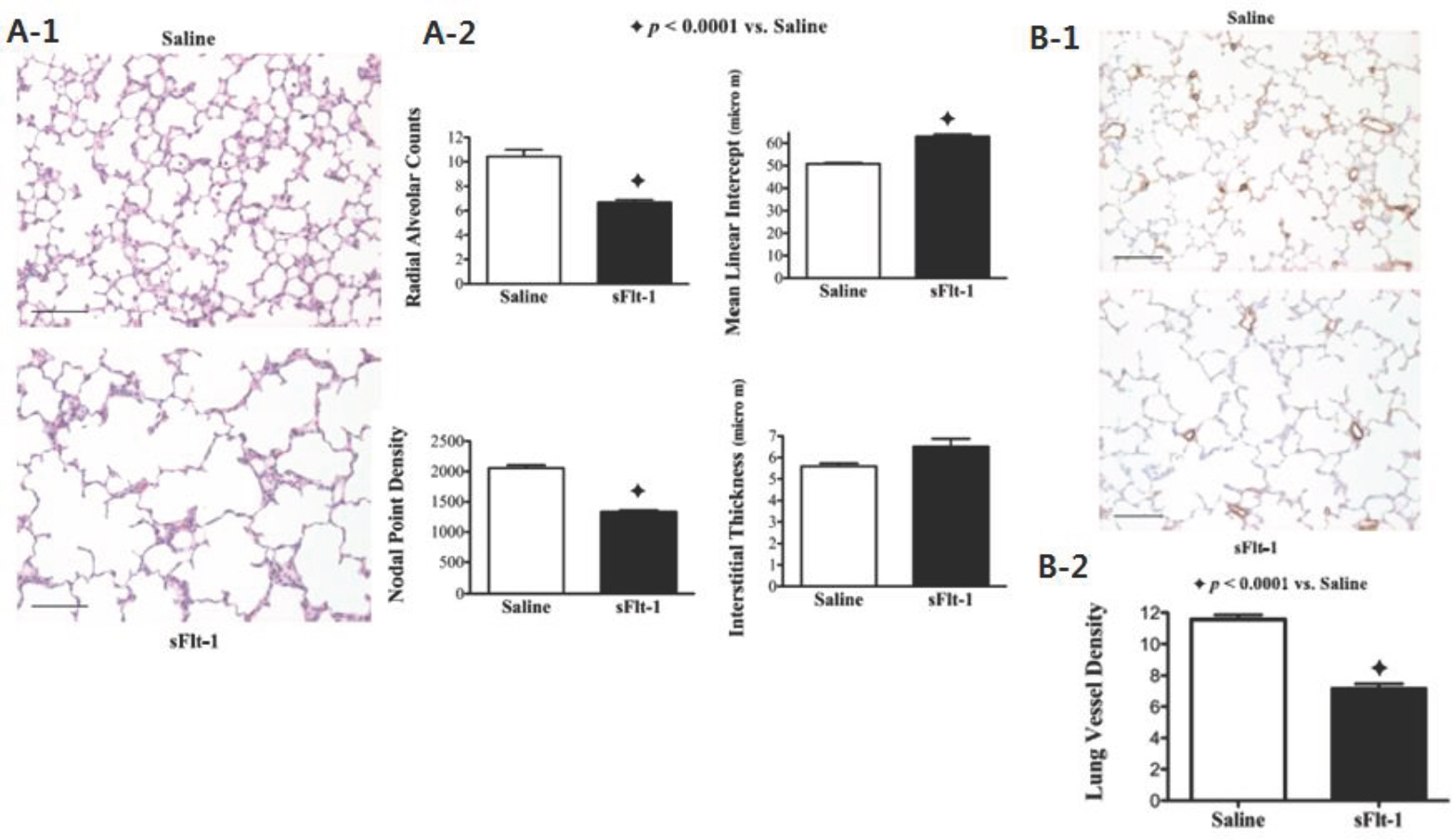
Article9. Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L36–46.
Article10. Eriksson L, Haglund B, Odlind V, Altman M, Kieler H. Prenatal inflammatory risk factors for development of bronchopulmonary dysplasia. Pediatr Pulmonol. 2014; 49:665–72.
Article11. National High Blood Pressure Education Program Working Group Report on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 1990; 163:1691–712.12. Shah DM. Hypertensive disorders of pregnancy. Martin RJ, Fanaroff AA, Walsh MC, editors. editors.Neonatal-perinatal medicine. 9th ed.St. Louis: Elsevier;2011. p277.
Article13. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003; 111:649–58.
Article14. Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005; 46:1077–85.
Article15. Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008; 294:H541–50.
Article16. Silasi M, Cohen B, Karumanchi S, Rana S. Abnormal placentation, angiogenic factors, and the pathogenesis of preeclampsia. Obstet Gynecol Clin N Am. 2010; 37:239–53.
Article17. Zavalza-Gómez AB. Obesity and oxidative stress: a direct link to preeclampsia? Arch Gynecol Obstet. 2011; 283:415–22.
Article18. Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001; 280:C1358–66.
Article19. Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Kar-panen T, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002; 160:1405–23.
Article20. Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003; 88:2348–51.
Article21. Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, et al. Overexpression of the soluble vascular endothelial growth factor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003; 88:5555–63.22. Veas CJ, Aguilera VC, Muñoz IJ, Gallardo VI, Miguel PL, González MA, et al. Fetal endothelium dysfunction is associated with circulating maternal levels of sE-selectin, sVCAM1, and sFlt-1 during preeclampsia. J Matern Fetal Neonatal Med. 2011; 24:1371–7.
Article23. Polliotti BM, Fry AG, Saller DN, Mooney RA, Cox C, Miller RK. Second-trimester maternal serum placental growth factor and vascular endothelial growth factor for predicting severe, early-onset preeclampsia. Obstet Gynecol. 2003; 101:1266–74.
Article24. Taylor RN, Grimwood J, Taylor RS, Mc-Master MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003; 188:177–82.
Article25. Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) and FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003; 21:60–5.26. Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003; 349:427–34.
Article27. Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massagué J, et al. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992; 267:19027–30.
Article28. Gougos A, St Jacques S, Greaves A, O'Connell PJ, d'Apice AJ, Bühring HJ, et al. Identification of distinct epitopes of endoglin, an RGD-containing glycoprotein of endothelial cells, leukemic cells, and syncytiotrophoblasts. Int Immunol. 1992; 4:83–92.
Article29. Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006; 12:642–9.
Article30. Tsao PN, Wei SC, Su YN, Chou HC, Chen CY, Hsieh WS. Excess soluble fms-like tyrosine kinase 1 and low platelet counts in premature neonates of preeclamptic mothers. Pediatrics. 2005; 116:468–72.
Article31. Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000; 106:1311–9.
Article32. Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001; 163:737–44.
Article33. Lassus P, Ristimaki A, Ylikorkala O, Viinikka L, Andersson S. Endothelial growth factor in human preterm lung. Am J Respir Crit Care Med. 1999; 159:1429–33.34. Janer J, Andersson S, Kajantie E, Lassus P. Endostatin concentration in cord plasma predicts the development of bronchopulmonary dysplasia in very low birth weight infants. Pediatrics. 2009; 123:1142–6.35. Yen TA, Yang HI, Hsieh WS, Chou HC, Chen CY, Tsou KI, et al. Taiwan Premature Infant Developmental Collaborative Study Group. Preeclampsia and the risk of bronchopulmonary dysplasia in VLBW infants: a population based study. PLoS One. 2013; 8:e75168.
Article36. O'Shea JE, Davis PG, Doyle LW; Victorian Infant Collaborative Study Group, Maternal preeclampsia and risk of bronchopulmonary dysplasia in preterm infants. Pediatr Res. 2012; 71:210–4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bronchopulmonary Dysplasia
- Radiological analysis of bronchopulmonary dysplasia
- Pathophysiology and Risk Factors of Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia
- Prevention of Bronchopulmonary Dysplasia
- A Case of Dexamethasone induced Hypertrophic Cardiomyopathy in Neonate with Bronchopulmonary Dysplasia