Diabetes Metab J.
2015 Feb;39(1):51-58. 10.4093/dmj.2015.39.1.51.
Hexane Extract of Orthosiphon stamineus Induces Insulin Expression and Prevents Glucotoxicity in INS-1 Cells
- Affiliations
-
- 1Department of Pediatrics, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 2Department of Physiology, Yeungnam University College of Medicine, Daegu, Korea. ywkim@med.yu.ac.kr
- 3Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea.
- 4Yeungnam University College of Pharmacy, Gyeongsan, Korea.
- KMID: 2280657
- DOI: http://doi.org/10.4093/dmj.2015.39.1.51
Abstract
- BACKGROUND
Hyperglycemia, a characteristic feature of diabetes, induces glucotoxicity in pancreatic beta-cells, resulting in further impairment of insulin secretion and worsening glycemic control. Thus, preservation of insulin secretory capacity is essential for the management of type 2 diabetes. In this study, we evaluated the ability of an Orthosiphon stamineus (OS) extract to prevent glucotoxicity in insulin-producing cells.
METHODS
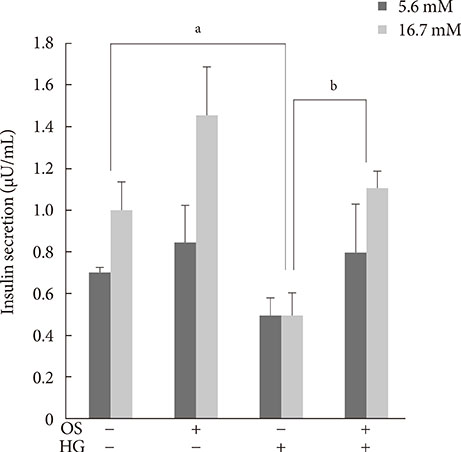
We measured insulin mRNA expression and glucose-stimulated insulin secretion (GSIS) in OS-treated INS-1 cells after exposure to a high glucose (HG; 30 mM) concentration.
RESULTS
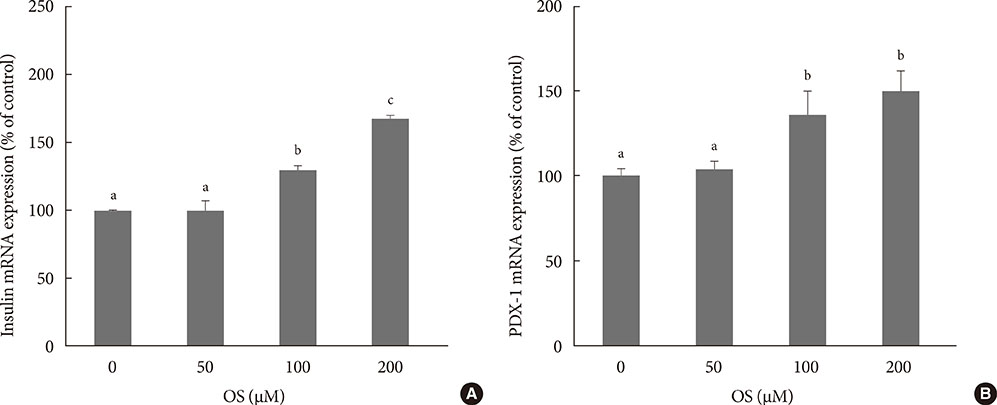
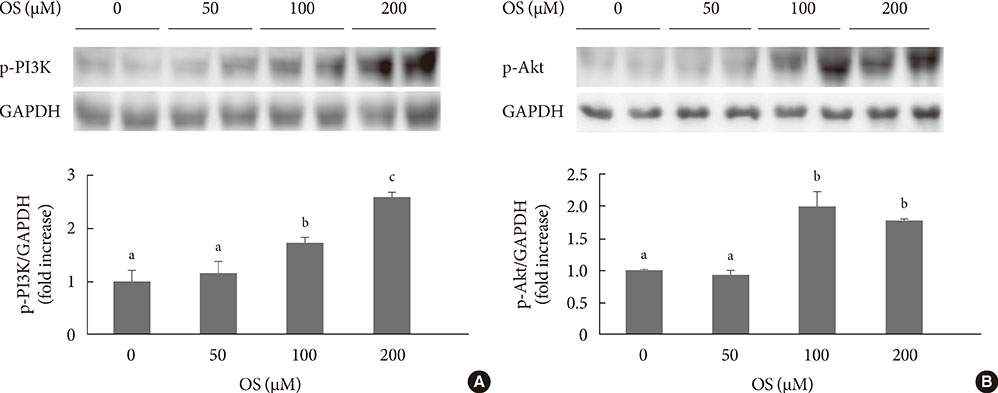
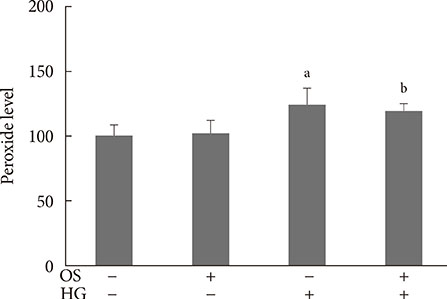
The hexane extract of OS elevated mRNA expression of insulin as well as pancreatic and duodenal homeobox-1 of INS-1 cells in a dose-dependent manner. The hexane OS extract also increased the levels of phosphorylated phosphatidylinositol 3-kinase (PI3K) in a concentration-dependent manner. Additionally, Akt phosphorylation was elevated by treatment with 100 and 200 micromol of the hexane OS extract. Three days of HG exposure suppressed insulin mRNA expression and GSIS; these expressions were restored by treatment with the hexane OS extract. HG elevated peroxide levels in the INS-1 cells. These levels were unaffected by OS treatment under both normal and hyperglycemic conditions.
CONCLUSION
Our results suggested that the hexane extract of OS elevates insulin mRNA expression and prevents glucotoxicity induced by a 3-day treatment with HG. This was associated with the activation of PI-3K and Akt.
MeSH Terms
Figure
Reference
-
1. Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008; 29:351–366.2. Won KC, Moon JS, Eun MJ, Yoon JS, Chun KA, Cho IH, Kim YW, Lee HW. A protective role for heme oxygenase-1 in INS-1 cells and rat islets that are exposed to high glucose conditions. J Korean Med Sci. 2006; 21:418–424.3. Poitout V, Robertson RP. Minireview: secondary beta-cell failure in type 2 diabetes: a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002; 143:339–342.4. Kim YW, Moon JS, Seo YJ, Park SY, Kim JY, Yoon JS, Lee IK, Lee HW, Won KC. Inhibition of fatty acid translocase cluster determinant 36 (CD36), stimulated by hyperglycemia, prevents glucotoxicity in INS-1 cells. Biochem Biophys Res Commun. 2012; 420:462–466.5. Ashcroft FM, Gribble FM. ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia. 1999; 42:903–919.6. Zawalich WS, Zawalich KC, Kelley GG. Regulation of insulin release by phospholipase C activation in mouse islets: differential effects of glucose and neurohumoral stimulation. Endocrinology. 1995; 136:4903–4909.7. Persaud SJ, Jones PM, Howell SL. Activation of protein kinase C is essential for sustained insulin secretion in response to cholinergic stimulation. Biochim Biophys Acta. 1991; 1091:120–122.8. Uchida T, Iwashita N, Ohara-Imaizumi M, Ogihara T, Nagai S, Choi JB, Tamura Y, Tada N, Kawamori R, Nakayama KI, Nagamatsu S, Watada H. Protein kinase Cdelta plays a non-redundant role in insulin secretion in pancreatic beta cells. J Biol Chem. 2007; 282:2707–2716.9. Hagman DK, Latour MG, Chakrabarti SK, Fontes G, Amyot J, Tremblay C, Semache M, Lausier JA, Roskens V, Mirmira RG, Jetton TL, Poitout V. Cyclical and alternating infusions of glucose and intralipid in rats inhibit insulin gene expression and Pdx-1 binding in islets. Diabetes. 2008; 57:424–431.10. Glauser DA, Schlegel W. The emerging role of FOXO transcription factors in pancreatic beta cells. J Endocrinol. 2007; 193:195–207.11. Furuya F, Shimura H, Asami K, Ichijo S, Takahashi K, Kaneshige M, Oikawa Y, Aida K, Endo T, Kobayashi T. Ligand-bound thyroid hormone receptor contributes to reprogramming of pancreatic acinar cells into insulin-producing cells. J Biol Chem. 2013; 288:16155–16166.12. Awale S, Tezuka Y, Banskota AH, Adnyana IK, Kadota S. Nitric oxide inhibitory isopimarane-type diterpenes from Orthosiphon stamineus of Indonesia. J Nat Prod. 2003; 66:255–258.13. Awale S, Tezuka Y, Banskota AH, Adnyana IK, Kadota S. Highly-oxygenated isopimarane-type diterpenes from Orthosiphon stamineus of Indonesia and their nitric oxide inhibitory activity. Chem Pharm Bull (Tokyo). 2003; 51:268–275.14. Sriplang K, Adisakwattana S, Rungsipipat A, Yibchok-Anun S. Effects of Orthosiphon stamineus aqueous extract on plasma glucose concentration and lipid profile in normal and streptozotocin-induced diabetic rats. J Ethnopharmacol. 2007; 109:510–514.15. Yam MF, Asmawi MZ, Basir R. An investigation of the anti-inflammatory and analgesic effects of Orthosiphon stamineus leaf extract. J Med Food. 2008; 11:362–368.16. Yam MF, Lim V, Salman IM, Ameer OZ, Ang LF, Rosidah N, Abdulkarim MF, Abdullah GZ, Basir R, Sadikun A, Asmawi MZ. HPLC and anti-inflammatory studies of the flavonoid rich chloroform extract fraction of Orthosiphon stamineus leaves. Molecules. 2010; 15:4452–4466.17. Doleckova I, Rarova L, Gruz J, Vondrusova M, Strnad M, Krystof V. Antiproliferative and antiangiogenic effects of flavone eupatorin, an active constituent of chloroform extract of Orthosiphon stamineus leaves. Fitoterapia. 2012; 83:1000–1007.18. Mohamed EA, Yam MF, Ang LF, Mohamed AJ, Asmawi MZ. Antidiabetic properties and mechanism of action of Orthosiphon stamineus Benth bioactive sub-fraction in streptozotocin-induced diabetic rats. J Acupunct Meridian Stud. 2013; 6:31–40.19. Son JY, Park SY, Kim JY, Won KC, Kim YD, Choi YJ, Zheng MS, Son JK, Kim YW. Orthosiphon stamineus reduces appetite and visceral fat in rats. J Korean Soc Appl Biol Chem. 2011; 54:200–205.20. Choi YJ, Park SY, Kim JY, Won KC, Kim BR, Son JK, Lee SH, Kim YW. Combined treatment of betulinic acid, a PTP1B inhibitor, with Orthosiphon stamineus extract decreases body weight in high-fat-fed mice. J Med Food. 2013; 16:2–8.21. Tajiri Y, Grill V. Aminoguanidine exerts a beta-cell function-preserving effect in high glucose-cultured beta-cells (INS-1). Int J Exp Diabetes Res. 2000; 1:111–119.22. Roger B, Papin J, Vacher P, Raoux M, Mulot A, Dubois M, Kerr-Conte J, Voy BH, Pattou F, Charpentier G, Jonas JC, Moustaid-Moussa N, Lang J. Adenylyl cyclase 8 is central to glucagon-like peptide 1 signalling and effects of chronically elevated glucose in rat and human pancreatic beta cells. Diabetologia. 2011; 54:390–402.23. McKinnon CM, Docherty K. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of beta cell identity and function. Diabetologia. 2001; 44:1203–1214.24. Andrali SS, Sampley ML, Vanderford NL, Ozcan S. Glucose regulation of insulin gene expression in pancreatic beta-cells. Biochem J. 2008; 415:1–10.25. Zhang SS, Hao E, Yu J, Liu W, Wang J, Levine F, Feng GS. Coordinated regulation by Shp2 tyrosine phosphatase of signaling events controlling insulin biosynthesis in pancreatic beta-cells. Proc Natl Acad Sci U S A. 2009; 106:7531–7536.26. Yang Y, Wang W, Liu Y, Guo T, Chen P, Ma K, Zhou C. alpha-lipoic acid inhibits high glucose-induced apoptosis in HIT-T15 cells. Dev Growth Differ. 2012; 54:557–565.27. Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, Han Z, Polonsky KS, Permutt MA. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004; 114:928–936.28. Wu H, MacFarlane WM, Tadayyon M, Arch JR, James RF, Docherty K. Insulin stimulates pancreatic-duodenal homoeobox factor-1 (PDX1) DNA-binding activity and insulin promoter activity in pancreatic beta cells. Biochem J. 1999; 344 Pt 3:813–818.29. Yam MF, Basir R, Asmawi MZ, Ismail Z. Antioxidant and hepatoprotective effects of Orthosiphon stamineus Benth: standardized extract. Am J Chin Med. 2007; 35:115–126.30. Koren-Gluzer M, Aviram M, Meilin E, Hayek T. The antioxidant HDL-associated paraoxonase-1 (PON1) attenuates diabetes development and stimulates beta-cell insulin release. Atherosclerosis. 2011; 219:510–518.31. Alshawsh MA, Abdulla MA, Ismail S, Amin ZA, Qader SW, Hadi HA, Harmal NS. Free radical scavenging, antimicrobial and immunomodulatory activities of Orthosiphon stamineus. Molecules. 2012; 17:5385–5395.32. Akowuah GA, Ismail Z, Norhayati I, Sadikun A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005; 93:311–317.33. Cumaoglu A, Ari N, Kartal M, Karasu C. Polyphenolic extracts from Olea europea L. protect against cytokine-induced beta-cell damage through maintenance of redox homeostasis. Rejuvenation Res. 2011; 14:325–334.34. Sumaryono W, Proksch P, Wray V, Witte L, Hartmann T. Qualitative and quantitative analysis of the phenolic constituents from Orthosiphon aristatus. Planta Med. 1991; 57:176–180.35. Tezuka Y, Stampoulis P, Banskota AH, Awale S, Tran KQ, Saiki I, Kadota S. Constituents of the Vietnamese medicinal plant Orthosiphon stamineus. Chem Pharm Bull (Tokyo). 2000; 48:1711–1719.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Padina arborescens extract protects high glucose-induced apoptosis in pancreatic beta cells by reducing oxidative stress
- The Glucotoxicity Protecting Effect of Ezetimibe in Pancreatic Beta Cells via Inhibition of CD36
- The Effect of Chronic High Glucose Concentration on Endoplasmic Reticulum Stress in INS-1 Cells
- The Effect of Tribbles-Related Protein 3 on ER Stress-Suppressed Insulin Gene Expression in INS-1 Cells
- A Portulaca oleracea L. extract promotes insulin secretion via a Kâº(ATP) channel dependent pathway in INS-1 pancreatic β-cells