Clin Exp Otorhinolaryngol.
2012 Mar;5(1):17-22.
Rate of Isolation and Trends of Antimicrobial Resistance of Multidrug Resistant Pseudomonas Aeruginosa from Otorrhea in Chronic Suppurative Otitis Media
- Affiliations
-
- 1Department of Otolaryngology, Kyung Hee University School of Medicine, Seoul, Korea. yeo2park@yahoo.co.kr
- 2Department of Obstetrics and Gynecology, The Catholic University of Korea School of Medicine, Suwon, Korea.
- 3Department of Otolaryngology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 4Department of Obstetrics and Gynecology, Kyiung Hee University School of Medicine, Seoul, Korea.
Abstract
OBJECTIVES
To assess the rate of isolation of Pseudomonas aeruginosa (PA) and multidrug-resistant PA (MDR-PA) from patients with chronic suppurative otitis media (CSOM) otorrhea and the annual trend of antibiotic-resistance.
METHODS
Otorrhea samples were collected aseptically from 1,598 CSOM patients. The rate of bacterial isolation and the results of antibiotic susceptibility testing were evaluated retrospectively.
RESULTS
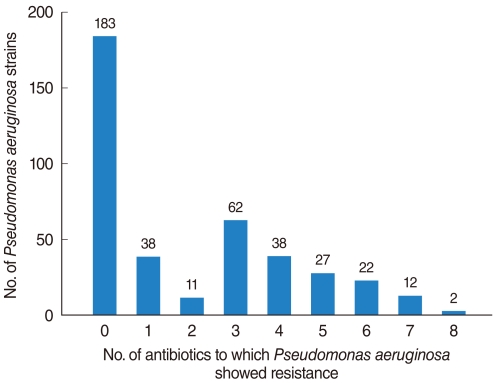
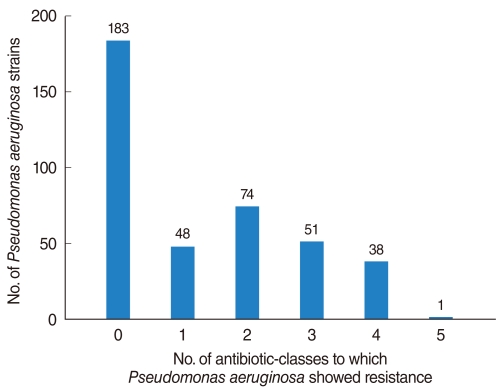
The PA isolation rate from CSOM otorrhea was 24.4%. Of the 398 isolated strains tested for their susceptibilities to 10 antibiotics, 395 strains showed definitive results. Of these, 183 (46.3%) were susceptible to whole antibiotics and 212 (53.7%) was resistant to more than 1 antibiotics, with the frequency of antibiotics-resistance increasing significantly over time. Although strains susceptible to all antibiotics decreased over time, the rate of isolation of MDR-PA did not change significantly. Resistance to aminoglycosides and quinolones was higher than to other antibiotics and significantly increased over time, whereas resistance to other antibiotics showed no trend.
CONCLUSION
MDR-PA, assessed using five individual antibiotics and six antibiotic-classes, showed no tendency to increase or decrease over time. This may have been due to increased concern about antibiotic-resistant bacterial strains, leading to improved infection control within hospitals and healthcare centers.
MeSH Terms
Figure
Reference
-
1. Bergogne-Berezin E. Cohen J, Powderly WG, editors. Pseudomonas and miscellaneous gram-negative bacilli. Infectious diseases. 2004. 2nd ed. Philadelphia, PA: Mosby;p. 2203–2226.2. Song W, Woo HJ, Kim JS, Lee KM. In vitro activity of beta-lactams in combination with other antimicrobial agents against resistant strains of Pseudomonas aeruginosa. Int J Antimicrob Agents. 2003; 1. 21(1):8–12. PMID: 12507832.3. Park DC, Lee SK, Cha CI, Lee SO, Lee MS, Yeo SG. Antimicrobial resistance of Staphylococcus from otorrhea in chronic suppurative otitis media and comparison with results of all isolated Staphylococci. Eur J Clin Microbiol Infect Dis. 2008; 7. 27(7):571–577. PMID: 18299908.
Article4. Wikler MA. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: sixteenth informational supplement. 2006. Wayne, PA: Clinical and Laboratory Standards Institute.5. Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002; 3. 01. 34(5):634–640. PMID: 11823954.
Article6. Pollack M. Mandell GL, Bennett JE, Dolin R, editors. Pseudomonas aeruginosa. Mandell, Douglas and Bennett's principles and practice of infectious diseases. 2000. 5th ed. Edinburgh, UK: Churchill Livingstone;p. 2310–2327.7. Jones RN, Kirby JT, Beach ML, Biedenbach DJ, Pfaller MA. Geographic variations in activity of broad-spectrum beta-lactams against Pseudomonas aeruginosa: summary of the worldwide SENTRY Antimicrobial Surveillance Program (1997-2000). Diagn Microbiol Infect Dis. 2002; 7. 43(3):239–243. PMID: 12106958.8. Walsh TR, Toleman MA, Hryniewicz W, Bennett PM, Jones RN. Evolution of an integron carrying blaVIM-2 in Eastern Europe: report from the SENTRY Antimicrobial Surveillance Program. J Antimicrob Chemother. 2003; 7. 52(1):116–119. PMID: 12805257.
Article9. Carmeli Y, Troillet N, Eliopoulos GM, Samore MH. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob Agents Chemother. 1999; 6. 43(6):1379–1382. PMID: 10348756.10. Llano-Sotelo B, Azucena EF Jr, Kotra LP, Mobashery S, Chow CS. Aminoglycosides modified by resistance enzymes display diminished binding to the bacterial ribosomal aminoacyl-tRNA site. Chem Biol. 2002; 4. 9(4):455–463. PMID: 11983334.
Article11. MacLeod DL, Nelson LE, Shawar RM, Lin BB, Lockwood LG, Dirk JE, et al. Aminoglycoside-resistance mechanisms for cystic fibrosis Pseudomonas aeruginosa isolates are unchanged by long-term, intermittent, inhaled tobramycin treatment. J Infect Dis. 2000; 3. 181(3):1180–1184. PMID: 10720551.12. Jo JT, Brinkman FS, Hancock RE. Aminoglycoside efflux in Pseudomonas aeruginosa: involvement of novel outer membrane proteins. Antimicrob Agents Chemother. 2003; 3. 47(3):1101–1111. PMID: 12604548.13. Doi Y, Arakawa Y. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin Infect Dis. 2007; 7. 01. 45(1):88–94. PMID: 17554708.
Article14. Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerg Infect Dis. 2001; Mar-Apr. 7(2):337–341. PMID: 11294736.
Article15. Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-oprM efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000; 12. 44(12):3322–3327. PMID: 11083635.16. Vahaboglu H, Hall LM, Mulazimoglu L, Dodanli S, Yildirim I, Livermore DM. Resistance to extended-spectrum cephalosporins, caused by PER-1 beta-lactamase, in Salmonella typhimurium from Istanbul, Turkey. J Med Microbiol. 1995; 10. 43(4):294–299. PMID: 7562992.17. Mugnier P, Dubrous P, Casin I, Arlet G, Collatz E. A TEM-derived extended-spectrum beta-lactamase in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996; 11. 40(11):2488–2493. PMID: 8913451.
Article18. Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991; 1. 35(1):147–151. PMID: 1901695.
Article19. Lee K, Kim YA, Park YJ, Lee HS, Kim MY, Kim EC, et al. Increasing prevalence of vancomycin-resistant enterococci, and cefoxitin-, imipenem- and fluoroquinolone-resistant gram-negative bacilli: a KONSAR study in 2002. Yonsei Med J. 2004; 8. 31. 45(4):598–608. PMID: 15344199.
Article20. Lee H, Yong D, Lee K, Hong SG, Kim EC, Jeong SH, et al. Antimicrobial resistance of clinically important bacteria isolated from 12 hospitals in Korea in 2004. Korean J Clin Microbiol. 2005; 4. 8(1):66–73.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bacteriology of Chronic Otitis Media: Changing of Detection Rate of Methicillin-Resistant Staphylococcus Aureus and Pseudomonas Aeruginosa
- Current Bacteriology of Chronic Suppurative Otitis Media
- Antimicrobial Resistance and Clones of Acinetobacter Species and Pseudomonas aeruginosa
- Sentinel Surveillance and Molecular Epidemiology of Multidrug Resistance Bacteria
- Microbiological Culture Sensitivity Profile of Chronic Otitis Media