Clin Exp Otorhinolaryngol.
2012 Apr;5(Suppl 1):S19-S23.
Magnetic Resonance Imaging Compatibility of the Polymer-based Cochlear Implant
- Affiliations
-
- 1School of Electrical Engineering and Computer Science, College of Engineering, Seoul National University, Seoul, Korea. kimsj@snu.ac.kr
- 2Inter-University Semiconductor Research Center, Seoul National University, Seoul, Korea.
- 3Research Institute, Nurobiosys Corporation, Suwon, Korea.
- 4Department of Electronics Engineering, College of Engineering, Ewha Womans University, Seoul, Korea.
- 5Department of Biomedical Engineering, College of Electronics & Information, Kyung Hee University, Yongin, Korea.
- 6Neuroscience Research Institute, Gachon University of Medicine and Science, Incheon, Korea.
Abstract
OBJECTIVES
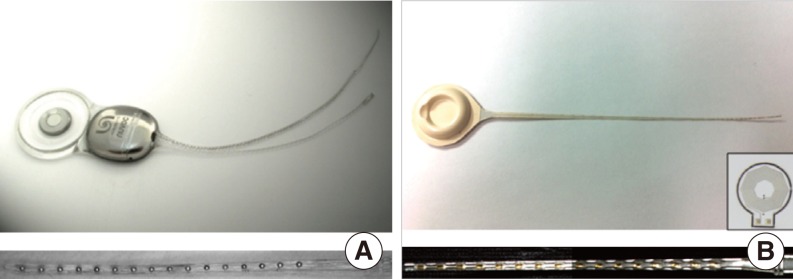
In this study, we compared the magnetic resonance (MR) image artifacts caused by a conventional metal-based cochlear implant and a newly developed liquid crystal polymer (LCP)-based device.
METHODS
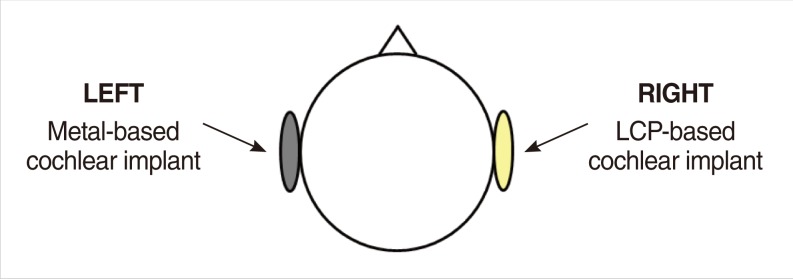
The metal-based cochlear implant system (Nurobiosys Co.) was attached to side of the head of a subject and the LCP-based device was attached to opposite side. In both devices, alignment magnets were removed for safety. Magnetic resonance imaging (MRI) was performed on a widely used 3.0 T and an ultra-high 7.0 T MRI machine. 3.0 and 7.0 T MR images were acquired using T1- and T2*-weighted gradient echo sequences, respectively.
RESULTS
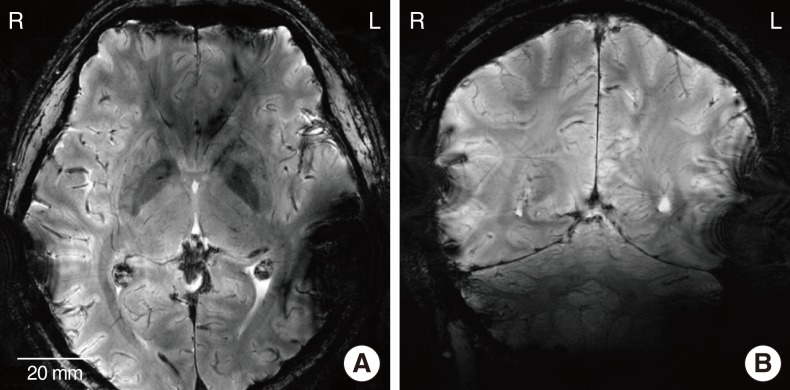
In the 3.0 T images, the metal-based device on the left side generated the significant amount of artifacts. The MR images in the proximity of the metal package were obscured by the artifacts in both axial and sagittal views. On the other hand, the MR images near the LCP-based device were relatively free from the artifacts and clearly showed the brain structures. 7.0 T MR images showed the more severe distortion in the both sides but the metal-based cochlear implant system caused a much larger obscure area than the LCP-based system.
CONCLUSION
The novel LCP-based cochlear implant provides a good MRI compatibility beyond present-day cochlear implants. Thus, MR images can be obtained from the subjects even with the implanted LCP-based neural prosthetic systems providing useful diagnostic information. Furthermore, it will be also useful for functional MRI studies of the auditory perception mechanism after cochlear implantations as well as for positron emission tomography-MRI hybrid imaging.
MeSH Terms
Figure
Reference
-
1. Wilson BS, Dorman MF. Cochlear implants: a remarkable past and a brilliant future. Hear Res. 2008; 8. 242(1-2):3–21. PMID: 18616994.
Article2. Gubbels SP, McMenomey SO. Safety study of the Cochlear Nucleus 24 device with internal magnet in the 1.5 Tesla magnetic resonance imaging scanner. Laryngoscope. 2006; 6. 116(6):865–871. PMID: 16735911.
Article3. Vincent C, Ruzza I, Vaneecloo FM, Dubrulle F. Magnetic resonance imaging with the Digisonic SP Neurelec cochlear implant. Eur Arch Otorhinolaryngol. 2008; 9. 265(9):1043–1046. PMID: 18297299.
Article4. Majdani O, Leinung M, Rau T, Akbarian A, Zimmerling M, Lenarz M, et al. Demagnetization of cochlear implants and temperature changes in 3.0T MRI environment. Otolaryngol Head Neck Surg. 2008; 12. 139(6):833–839. PMID: 19041512.
Article5. Majdani O, Rau TS, Gotz F, Zimmerling M, Lenarz M, Lenarz T, et al. Artifacts caused by cochlear implants with non-removable magnets in 3T MRI: phantom and cadaveric studies. Eur Arch Otorhinolaryngol. 2009; 12. 266(12):1885–1890. PMID: 19629509.
Article6. Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007; 1. 27. 369(9558):293–298. PMID: 17258669.
Article7. Lee SW, Min KS, Jeong J, Kim J, Kim SJ. Monolithic encapsulation of implantable neuroprosthetic devices using liquid crystal polymers. IEEE Trans Biomed Eng. 2011; 8. 58(8):2255–2263.8. Hassler C, Boretius T, Stieglitz T. Polymers for neural implants. J Polym Sci B Polym Phys. 2011; 1. 49(1):18–33.
Article9. Lee SW, Seo JM, Ha S, Kim ET, Chung H, Kim SJ. Development of microelectrode arrays for artificial retinal implants using liquid crystal polymers. Invest Ophthalmol Vis Sci. 2009; 12. 50(12):5859–5866. PMID: 19553608.
Article10. Frisk L, Ristolainen E. Flip chip attachment on flexible LCP substrate using an ACF. Microelectron Reliab. 2005; Mar-Apr. 45(3-4):583–588.
Article11. An SK, Park SI, Jun SB, Lee CJ, Byun KM, Sung JH, et al. Design for a simplified cochlear implant system. IEEE Trans Biomed Eng. 2007; 6. 54(6 Pt 1):973–982. PMID: 17554817.
Article12. Jeong J, Lee SW, Min KS, Eom K, Bae SH, Kim SJ. Eye-surface conformable telemetric structure for polymer-based retinal prosthesis. 33rd Annual International IEEE EMBS Conference. 2011 Aug 30 - Sep 3; Boston, MA, USA. p. 1097–1100.
Article13. Cho ZH, Son YD, Kim HK, Kim KN, Oh SH, Han JY, et al. A fusion PET-MRI system with a high-resolution research tomograph-PET and ultra-high field 7.0 T-MRI for the molecular-genetic imaging of the brain. Proteomics. 2008; 3. 8(6):1302–1323. PMID: 18338828.14. Bhidayasiri R, Bronstein JM, Sinha S, Krahl SE, Ahn S, Behnke EJ, et al. Bilateral neurostimulation systems used for deep brain stimulation: in vitro study of MRI-related heating at 1.5 T and implications for clinical imaging of the brain. Magn Reson Imaging. 2005; 5. 23(4):549–555. PMID: 15919600.
Article15. Tagliati M, Jankovic J, Pagan F, Susatia F, Isaias IU, Okun MS, et al. Safety of MRI in patients with implanted deep brain stimulation devices. Neuroimage. 2009; 8. 47(Suppl 2):T53–T57. PMID: 19376247.
Article16. Mohsin SA, Sheikh NM, Maalik A. The effect of the MRI radiofrequency field on medical leads embedded beneath skin tissue. 2010 International Conference on Electromagnetics in Advanced Applications (ICEAA). 2010 Sep 20-24; Sydney, Australia. p. 513–516.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Magnetic Resonance Imaging in Cochlear Implant Patient
- A Case of Cochlear Implantation in the Patient With Neurofibromatosis Type II Considering Magnetic Resonance Imaging
- Auditory Rehabilitation - Cochlear Implantation
- A Case with the Bilateral Narrow Bony Cochlear Nerve Canals Associated with Near Normal Hearing Thresholds
- Cochlear Implantation in the Cochlear Nerve Hypoplasia or Aplasia as Suggested by Temporal Bone Magnetic Resonance Imaging