Clin Exp Otorhinolaryngol.
2013 Dec;6(4):219-225.
Protective Role of Trimetazidine Against Neomycin-induced Hair Cell Damage in Zebrafish
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College of Medicine, Seoul, Korea. mednlaw@korea.ac.kr
- 2Laboratory of Neurodevelopmental Genetics, Graduate School of Medicine, Korea University, Seoul, Korea.
Abstract
OBJECTIVES
Trimetazidine (TMZ) is known to reduce the generation of oxygen-derived free radicals. The objective of the present study was to evaluate the effects of TMZ on neomycin-induced ototoxicity in transgenic zebrafish (Brn3C: EGFP).
METHODS
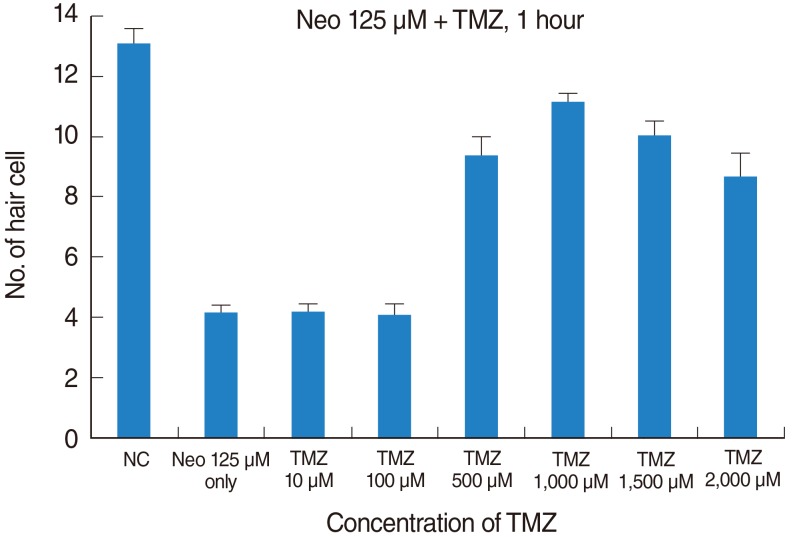
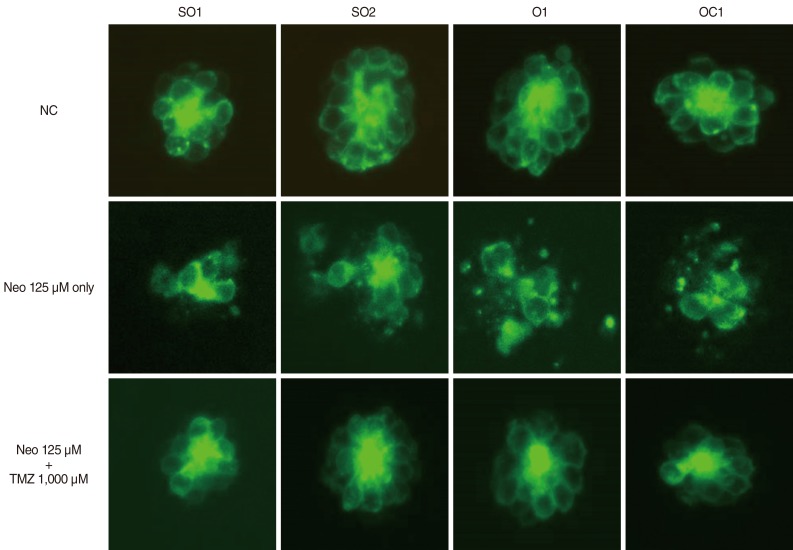
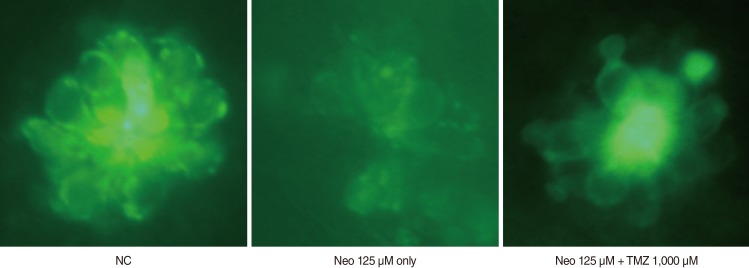
Five-day, postfertilization zebrafish larvae were exposed to 125 microM neomycin and one of the following TMZ concentrations for 1 hour: 10 microM, 100 microM, 500 microM, 1,000 microM, 1,500 microM, or 2,000 microM. Hair cells within the neuromasts of the supraorbital (SO1 and SO2), otic (O1), and occipital (OC1) lateral lines were analyzed using fluorescence microscopy and confocal microscopy (n=10). Hair cell survival was calculated as a percentage of hair cells in the control group that were not exposed to neomycin. Ultrastructural changes were evaluated using scanning electron microscopy.
RESULTS
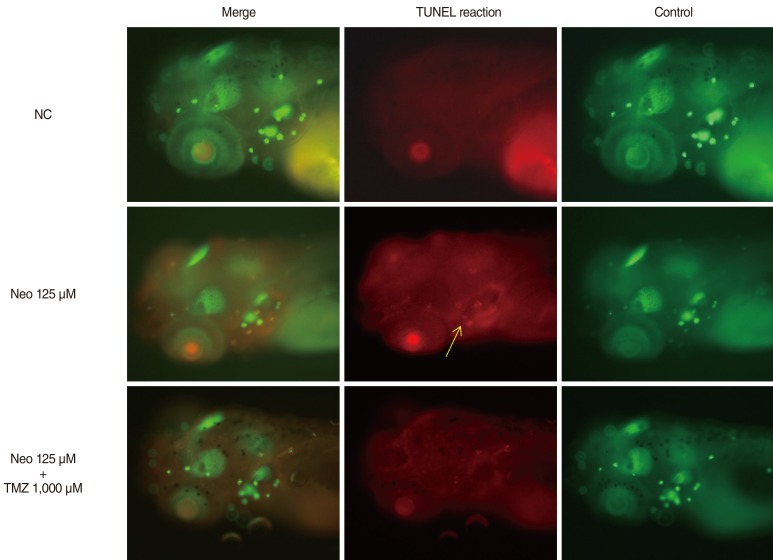
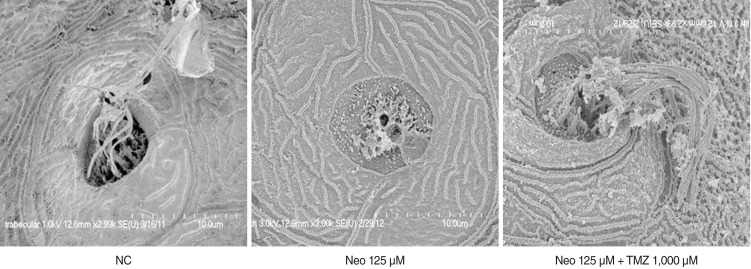
TMZ protected against neomycin-induced hair cell loss in the neuromasts (TMZ 1,000 microM, 11.2+/-0.4 cells; 125 microM neomycin only, 4.2+/-0.5 cells; n=10; P<0.05) and decreased the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) reaction. In the ultrastructural analysis, structures of mitochondria and hair cells within the neuromasts were preserved in zebrafish exposed to 125 microM neomycin and 1,000 microM TMZ.
CONCLUSION
TMZ attenuated neomycin-induced hair cell loss in zebrafish. The results of this study suggest that neomycin induces apoptosis, and that apoptotic cell death can be prevented by treatment with tremetazidine.
Keyword
MeSH Terms
Figure
Reference
-
1. Warchol ME. Cellular mechanisms of aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 2010; 10. 18(5):454–458. PMID: 20717031.
Article2. Rizzi MD, Hirose K. Aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 2007; 10. 15(5):352–357. PMID: 17823553.
Article3. Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007; 1. 13(1):119–126. PMID: 17266591.
Article4. Xie J, Talaska AE, Schacht J. New developments in aminoglycoside therapy and ototoxicity. Hear Res. 2011; 11. 281(1-2):28–37. PMID: 21640178.
Article5. Jung HH, Chang J, Yang JY, Choi J, Im GJ, Chae SW. Protective role of antidiabetic drug metformin against gentamicin induced apoptosis in auditory cell line. Hear Res. 2011; 12. 282(1-2):92–96. PMID: 21979311.
Article6. Rybak LP, Kelly T. Ototoxicity: bioprotective mechanisms. Curr Opin Otolaryngol Head Neck Surg. 2003; 10. 11(5):328–333. PMID: 14502062.
Article7. Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000; Jan-Feb. 5(1):3–22. PMID: 10686428.
Article8. De la Cruz Rodriguez LA, Araujo CR, Posleman SE, Rey MR. Attenuation of gentamicin-induced nephrotoxicity: trimetazidine versus N-acetyl cysteine. J Appl Toxicol. 2010; 5. 30(4):343–353. PMID: 20063365.9. Singh D, Chander V, Chopra K. Carvedilol and trimetazidine attenuates ferric nitrilotriacetate-induced oxidative renal injury in rats. Toxicology. 2003; 9. 191(2-3):143–151. PMID: 12965117.
Article10. Unal OF, Ghoreishi SM, Atas A, Akyurek N, Akyol G, Gursel B. Prevention of gentamicin induced ototoxicity by trimetazidine in animal model. Int J Pediatr Otorhinolaryngol. 2005; 2. 69(2):193–199. PMID: 15656952.11. Guarnieri C, Muscari C. Beneficial effects of trimetazidine on mitochondrial function and superoxide production in the cardiac muscle. Cardiovasc Drugs Ther. 1990; 4(Suppl 4):814–815. PMID: 1965528.
Article12. Ou HC, Raible DW, Rubel EW. Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hear Res. 2007; 11. 233(1-2):46–53. PMID: 17709218.
Article13. Ton C, Parng C. The use of zebrafish for assessing ototoxic and otoprotective agents. Hear Res. 2005; 10. 208(1-2):79–88. PMID: 16014323.
Article14. Ou HC, Cunningham LL, Francis SP, Brandon CS, Simon JA, Raible DW, et al. Identification of FDA-approved drugs and bioactives that protect hair cells in the zebrafish (Danio rerio) lateral line and mouse (Mus musculus) utricle. J Assoc Res Otolaryngol. 2009; 6. 10(2):191–203. PMID: 19241104.
Article15. Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005; 1. 4(1):35–44. PMID: 15688071.
Article16. Choi J, Im GJ, Chang J, Chae SW, Lee SH, Kwon SY, et al. Protective effects of apocynin on cisplatin-induced ototoxicity in an auditory cell line and in zebrafish. J Appl Toxicol. 2013; 2. 33(2):125–133. PMID: 22147442.
Article17. Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000; 5. 421(2):189–198. PMID: 10813781.
Article18. Kim TY, Son SW, Choi BJ, Park CH. A study of structure of the sucker of Common freshwater goby (Rhinogobius brunneus) and Triden goby (Tridentiger brevispinis). Korean J Electron Microsc. 2002; 3. 32(1):57–66.19. Priuska EM, Schacht J. Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochem Pharmacol. 1995; 11. 50(11):1749–1752. PMID: 8615852.
Article20. Williams FM, Tanda K, Kus M, Williams TJ. Trimetazidine inhibits neutrophil accumulation after myocardial ischaemia and reperfusion in rabbits. J Cardiovasc Pharmacol. 1993; 12. 22(6):828–833. PMID: 7509900.
Article21. El Banani H, Bernard M, Baetz D, Cabanes E, Cozzone P, Lucien A, et al. Changes in intracellular sodium and pH during ischaemia-reperfusion are attenuated by trimetazidine: comparison between low- and zero-flow ischaemia. Cardiovasc Res. 2000; 9. 47(4):688–696. PMID: 10974217.
Article22. Onbasili AO, Yeniceriglu Y, Agaoglu P, Karul A, Tekten T, Akar H, et al. Trimetazidine in the prevention of contrast-induced nephropathy after coronary procedures. Heart. 2007; 6. 93(6):698–702. PMID: 17065180.
Article23. Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci U S A. 2000; 11. 97(24):12965–12969. PMID: 11087852.
Article24. Ou H, Simon JA, Rubel EW, Raible DW. Screening for chemicals that affect hair cell death and survival in the zebrafish lateral line. Hear Res. 2012; 6. 288(1-2):58–66. PMID: 22310494.
Article25. Sachidanandan C, Yeh JR, Peterson QP, Peterson RT. Identification of a novel retinoid by small molecule screening with zebrafish embryos. PLoS One. 2008; 4. 3(4):e1947. PMID: 18398471.
Article26. Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003; 3. 107(10):1355–1358. PMID: 12642353.
Article27. White RM, Cech J, Ratanasirintrawoot S, Lin CY, Rahl PB, Burke CJ, et al. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature. 2011; 3. 471(7339):518–522. PMID: 21430780.
Article28. Kari G, Rodeck U, Dicker AP. Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharmacol Ther. 2007; 7. 82(1):70–80. PMID: 17495877.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- microRNA-183 is Essential for Hair Cell Regeneration after Neomycin Injury in Zebrafish
- Minocycline Protects Vestibular Hair Cells from Neomycin-Induced Ototoxicity by Inhibiting Reactive Oxygen Species Production and Caspase-3 Activity
- Impact of Nicotine Exposure on Hair Cell Toxicity and Embryotoxicity During Zebrafish Development
- Update of Research on Aminoglycoside Ototoxicity
- Zebrafish and Mycobacterial infection