Clin Exp Otorhinolaryngol.
2014 Jun;7(2):106-111.
Effects of Topical Intranasal Doxycycline Treatment in the Rat Allergic Rhinitis Model
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Private Gazi Hospital, Izmir, Turkey. ozgur_tr@hotmail.com
- 2Department of Histology and Embriyology, Dokuz Eylul University School of Medicine, Izmir, Turkey.
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Dokuz Eylul University School of Medicine, Izmir, Turkey.
Abstract
OBJECTIVES
Allergic rhinitis (AR) is a chronic upper respiratory tract disease that inflames the mucous membranes of the nose and occurs when circulating inflammatory cells including eosinophils and basophils migrate to and accumulate in the inflammation area by passing through the interstitium and capillary walls. To pass through these barriers, the inflammatory cells degrade extracellular matrix proteins. Matrix metalloproteinases (MMPs) released by inflammatory cells mediate the degradation of these proteins. MMPs have synthetic inhibitors and doxycycline, a tetracycline antibiotic, inhibits MMPs. This study investigated the efficiency of intranasal doxycycline in decreasing the symptoms and inflammatory cell infiltration in an animal model of AR.
METHODS
AR was created in female Wistar rats by repeated intranasal challenge with ovalbumin by intraperitoneal injection. For 15 days, topical intranasal doxycycline was administered one hour before ovalbumin administration. Following intranasal administration, nasal symptoms were scored and the nasal mucosae of all rats were evaluated histopathologically. To investigate tissue changes, hematoxyline-eosin and Alcian blue/periodic acid Schiff stains were used. As well, cilia loss, goblet cell changes, vascular congestion, vascular proliferation, inflammatory cell infiltration, eosinophil infiltration and the degree of hypertrophy in chondrocytes were evaluated with light microscopy.
RESULTS
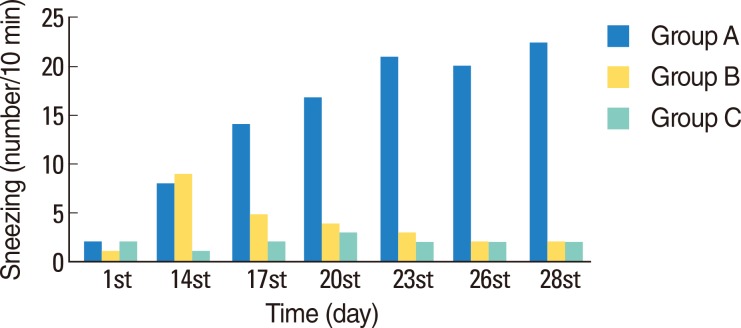
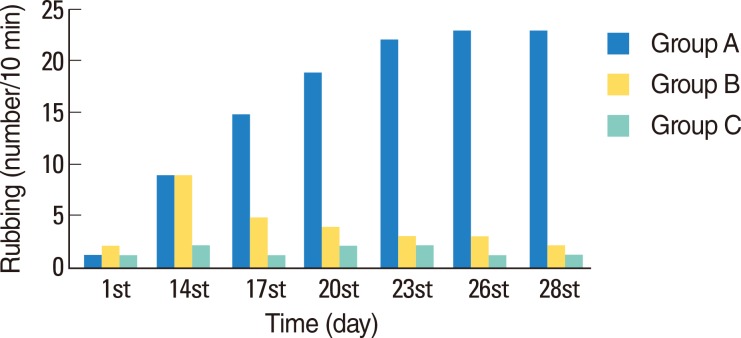
Typical symptoms of AR were decreased by intranasal doxycycline administration. These effects were stable after repeated intranasal ovalbumin administration. Histological evaluation of doxycycline treated rats did not reveal typical inflammatory changes associated with AR.
CONCLUSION
MMPs may have crucial functions in AR and topical intranasal doxycycline, which decreases inflammatory cell infiltration, may offer an alternative therapy for AR.
MeSH Terms
-
Administration, Intranasal
Animals
Basophils
Capillaries
Chondrocytes
Cilia
Coloring Agents
Doxycycline*
Eosinophils
Estrogens, Conjugated (USP)
Extracellular Matrix Proteins
Female
Goblet Cells
Humans
Hypertrophy
Inflammation
Injections, Intraperitoneal
Matrix Metalloproteinase Inhibitors
Matrix Metalloproteinases
Microscopy
Models, Animal
Mucous Membrane
Nasal Mucosa
Nose
Ovalbumin
Rats*
Rats, Wistar
Respiratory Tract Diseases
Rhinitis*
Tetracycline
Coloring Agents
Doxycycline
Estrogens, Conjugated (USP)
Extracellular Matrix Proteins
Matrix Metalloproteinase Inhibitors
Matrix Metalloproteinases
Ovalbumin
Tetracycline
Figure
Reference
-
1. Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998; 11. 12(2):12–26. PMID: 9972117.
Article2. Asano K, Kanai KI, Suzaki H. Suppressive activity of fexofenadine hydrochloride on metalloproteinase production from nasal fibroblasts in vitro. Clin Exp Allergy. 2004; 12. 34(12):1890–1898. PMID: 15663564.
Article3. De S, Fenton JE, Jones AS. Matrix metalloproteinases and their inhibitors innon-neoplastic otorhinolaryngological disease. J Laryngol Otol. 2005; 6. 119(6):436–442. PMID: 15992468.4. Lee KS, Jin SM, Kim HJ, Lee YC. Matrix metalloproteinase inhibitor regulatesinflammatory cell migration by reducing ICAM-1 and VCAM-1 expression in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol. 2003; 6. 111(6):1278–1284. PMID: 12789230.5. Baltacıoglu E, Akalın A. Tetracyclines and their non-antimicrobial properties: a new approach to their use in periodontal treatment. Hacettepe Dişhekimliği Fakültesi Dergisi. 2006; 30(1):97–107.6. Wen WD, Yuan F, Wang JL, Hou YP. Botulinum toxin therapy in the ovalbumin-sensitized rat. Neuroimmunomodulation. 2007; 14(2):78–83. PMID: 17713354.
Article7. Salib RJ, Howarth PH. Remodelling of the upper airways in allergic rhinitis: is it a feature of the disease? Clin Exp Allergy. 2003; 12. 33(12):1629–1633. PMID: 14656347.
Article8. Bousquet J, Jacot W, Vignola AM, Bachert C, Van Cauwenberge P. Allergic rhinitis: a disease remodeling the upper airways? J Allergy Clin Immunol. 2004; 1. 113(1):43–49. PMID: 14713906.9. Ercan I, Cakir BO, Basak T, Ozbal EA, Sahin A, Balci G, et al. Effects oftopical application of methotrexate on nasal mucosa in rats: a preclinicalassessment study. Otolaryngol Head Neck Surg. 2006; 5. 134(5):751–755. PMID: 16647529.10. Shimizu S, Hattori R, Majima Y, Shimizu T. Th2 cytokine inhibitor suplatasttosilate inhibits antigen-induced mucus hypersecretion in the nasal epithelium of sensitized rats. Ann Otol Rhinol Laryngol. 2009; 1. 118(1):67–72. PMID: 19244966.11. Bentley AM, Jacobson MR, Cumberworth V, Barkans JR, Moqbel R, Schwartz LB, et al. Immunohistology of the nasal mucosa in seasonal-allergic rhinitis: increases in activated eosinophils and epithelial mast cells. J Allergy Clin Immunol. 1992; 4. 89(4):877–883. PMID: 1532808.12. Herouy Y, Mellios P, Bandemir E, Dichmann S, Nockowski P, Schopf E, et al. Inflammation in stasis dermatitis upregulates MMP-1, MMP-2 and MMP-13 expression. J Dermatol Sci. 2001; 4. 25(3):198–205. PMID: 11240267.
Article13. Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, et al. Eosinophils as a source of matrix metalloproteinase-9 in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1997; 3. 16(3):212–219. PMID: 9070604.
Article14. Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol. 1997; 10. 17(4):519–528. PMID: 9376127.15. Nakaya M, Dohi M, Okunishi K, Nakagome K, Tanaka R, Imamura M, et al. Prolonged allergen challenge in murine nasal allergic rhinitis: nasal airway remodeling and adaptation of nasal airway responsiveness. Laryngoscope. 2007; 5. 117(5):881–885. PMID: 17473688.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allergic Rhinitis Mouse Model
- Management of Rhinitis: Allergic and Non-Allergic
- The Effect of the Combined Use of Immunotherapy and Topical Steroid on the Quality of Life in Allergic Rhinitis Patients
- Understanding the Mouse Model of Respiratory Allergic Diseases
- Intranasal Phototherapy in the Patients with Perennial Allergic Rhinitis