Clin Exp Otorhinolaryngol.
2014 Dec;7(4):286-294. 10.3342/ceo.2014.7.4.286.
Protective Effect of Metformin on Gentamicin-Induced Vestibulotoxicity in Rat Primary Cell Culture
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Korea University College of Medicine, Seoul, Korea. logopas@korea.ac.kr
- KMID: 2278369
- DOI: http://doi.org/10.3342/ceo.2014.7.4.286
Abstract
OBJECTIVES
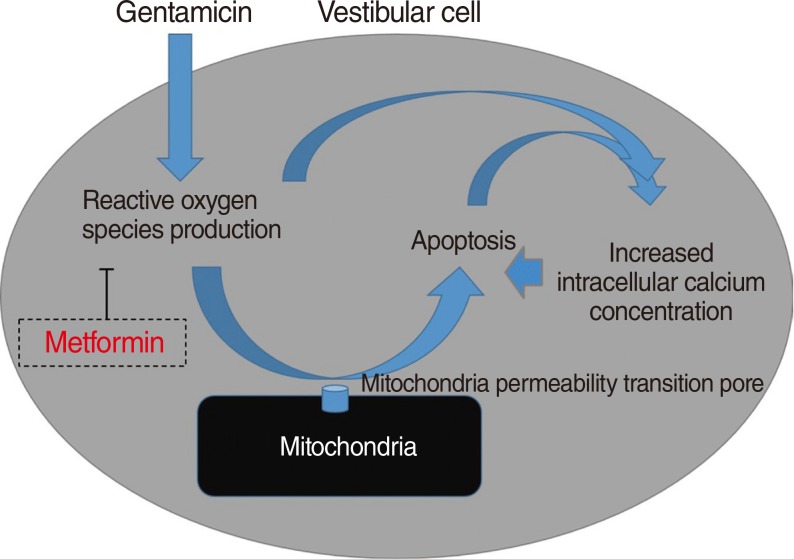
One of the antidiabetic drugs, metformin, have shown that it prevented oxidative stress-induced death in several cell types through a mechanism involving the opening of the permeability transition pore and cytochrome c release. Thus, it is possible that the antioxidative effect of metformin can also serve as protection against gentamicin-induced cytotoxicity related to reactive oxygen species (ROS). The aim of this study was to examine the protective effect of metformin on gentamicin-induced vestibulotoxicity in primary cell culture derived from rat utricle.
METHODS
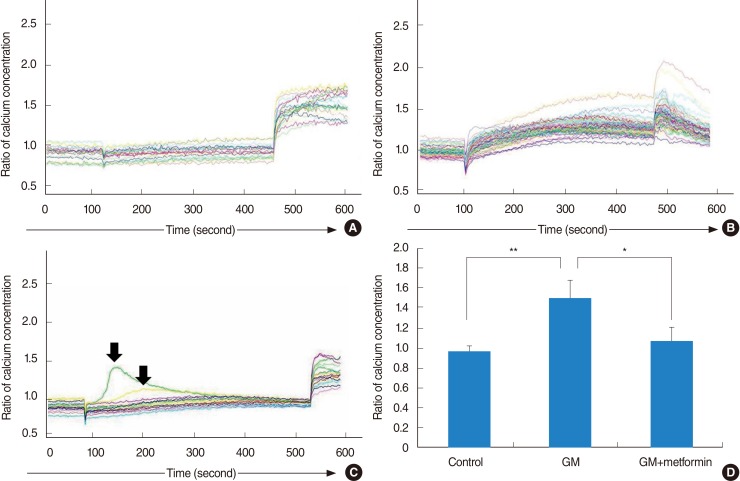
For vestibular primary cell culture, rat utricles were dissected and incubated. Gentamicin-induced cytotoxicity was measured in both the auditory and vestibular cells. To examine the effects of metformin on gentamicin-induced cytotoxicity in the primary cell culture, the cells were pretreated with metformin at a concentration of 1 mM for 24 hours, and then exposed to 2.5 mM gentamicin for 48 hours. The intracellular ROS level was measured using a fluorescent dye, and also measured using a FACScan flow cytometer. Intracellular calcium levels in the vestibular cells were measured with calcium imaging using Fura-2 AM.
RESULTS
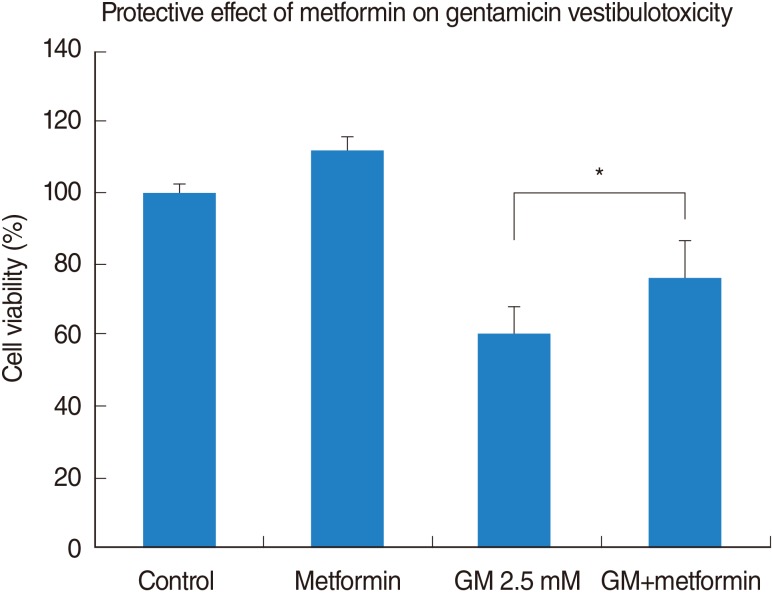
Vestibular cells were more sensitive to gentamicin-induced cytotoxicity than auditory hair cells. Metformin protects against gentamicin-induced cytotoxicity in vestibular cells. Metformin significantly reduced a gentamicin-induced increase in ROS, and also reduced an increase in intracellular calcium concentrations in gentamicin-induced cytotoxicity.
CONCLUSION
Metformin significantly reduced a gentamicin-induced increase in ROS, stabilized the intracellular calcium concentration, and inhibited gentamicin-induced apoptosis. Thus, Metformin showed protective effect on gentamicin-induced cytotoxicity in vestibular primary cell culture.
MeSH Terms
Figure
Reference
-
1. Sajjadi H, Paparella MM. Meniere's disease. Lancet. 2008; 8. 372(9636):406–414. PMID: 18675691.
Article2. Schuknecht HF. Ablation therapy in the management of Meniere's disease. Acta Otolaryngol Suppl. 1957; 132:1–42. PMID: 13457879.3. Pullens B, van Benthem PP. Intratympanic gentamicin for Meniere's disease or syndrome. Cochrane Database Syst Rev. 2011; 3. (3):CD008234. PMID: 21412917.4. Huon LK, Fang TY, Wang PC. Outcomes of intratympanic gentamicin injection to treat Meniere's disease. Otol Neurotol. 2012; 7. 33(5):706–714. PMID: 22699980.5. Blakley BW. Update on intratympanic gentamicin for Meniere's disease. Laryngoscope. 2000; 2. 110(2):236–240. PMID: 10680922.
Article6. Minor LB. Intratympanic gentamicin for control of vertigo in Meniere's disease: vestibular signs that specify completion of therapy. Am J Otol. 1999; 3. 20(2):209–219. PMID: 10100525.7. Pender DJ. Gentamicin tympanoclysis: effects on the vestibular secretory cells. Am J Otolaryngol. 1985; Sep-Oct. 6(5):358–367. PMID: 3907388.
Article8. Beck C. Intratympanic application of gentamicin for treatment of Meniere's disease. Keio J Med. 1986; 3. 35(1):36–41. PMID: 3747323.
Article9. Tange RA, Conijn EA, van Zeijl LG, Huizing EH. Pattern of gentamicin-induced cochlear degeneration in the guinea pig: a morphological and electrophysiological study. Arch Otorhinolaryngol. 1982; 236(2):173–184. PMID: 7150082.10. Quaranta A, Scaringi A, Aloidi A, Quaranta N, Salonna I. Intratympanic therapy for Meniere's disease: effect of administration of low concentration of gentamicin. Acta Otolaryngol. 2001; 4. 121(3):387–392. PMID: 11425206.11. Dehne N, Rauen U, de Groot H, Lautermann J. Involvement of the mitochondrial permeability transition in gentamicin ototoxicity. Hear Res. 2002; 7. 169(1-2):47–55. PMID: 12121739.
Article12. Jacotot E, Costantini P, Laboureau E, Zamzami N, Susin SA, Kroemer G. Mitochondrial membrane permeabilization during the apoptotic process. Ann N Y Acad Sci. 1999; 887:18–30. PMID: 10668461.
Article13. Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death: mechanistic aspects and methodological issues. Eur J Biochem. 1999; 9. 264(3):687–701. PMID: 10491114.
Article14. Brustovetsky N, Brustovetsky T, Purl KJ, Capano M, Crompton M, Dubinsky JM. Increased susceptibility of striatal mitochondria to calcium-induced permeability transition. J Neurosci. 2003; 6. 23(12):4858–4867. PMID: 12832508.
Article15. Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006; 5. 273(10):2077–2099. PMID: 16649987.
Article16. Fontaine E, Eriksson O, Ichas F, Bernardi P. Regulation of the permeability transition pore in skeletal muscle mitochondria: modulation by electron flow through the respiratory chain complex I. J Biol Chem. 1998; 5. 273(20):12662–12668. PMID: 9575229.17. Hirose K, Westrum LE, Stone JS, Zirpel L, Rubel EW. Dynamic studies of ototoxicity in mature avian auditory epithelium. Ann N Y Acad Sci. 1999; 11. 884:389–409. PMID: 10842609.
Article18. Ding D, Stracher A, Salvi RJ. Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res. 2002; 2. 164(1-2):115–126. PMID: 11950531.19. El-Mir MY, Detaille D, R-Villanueva G, Delgado-Esteban M, Guigas B, Attia S, et al. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci. 2008; 34(1):77–87. PMID: 18040888.
Article20. Guigas B, Detaille D, Chauvin C, Batandier C, De Oliveira F, Fontaine E, et al. Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological in vitro study. Biochem J. 2004; 9. 382(Pt 3):877–884. PMID: 15175014.
Article21. Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006; 1. 55(1):120–127. PMID: 16380484.
Article22. Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, et al. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun. 2010; 5. 396(2):199–205. PMID: 20398632.
Article23. Algire C, Moiseeva O, Deschenes-Simard X, Amrein L, Petruccelli L, Birman E, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila). 012; 4. 5(4):536–543. PMID: 22262811.
Article24. Im GJ, Chang JW, Choi J, Chae SW, Ko EJ, Jung HH. Protective effect of Korean red ginseng extract on cisplatin ototoxicity in HEI-OC1 auditory cells. Phytother Res. 2010; 4. 24(4):614–621. PMID: 20020438.
Article25. Chang J, Jung HH, Yang JY, Choi J, Im GJ, Chae SW. Protective role of antidiabetic drug metformin against gentamicin induced apoptosis in auditory cell line. Hear Res. 2011; 12. 282(1-2):92–96. PMID: 21979311.26. Choi J, Im GJ, Chang J, Chae SW, Lee SH, Kwon SY, et al. Protective effects of apocynin on cisplatin-induced ototoxicity in an auditory cell line and in zebrafish. J Appl Toxicol. 2013; 2. 33(2):125–133. PMID: 22147442.
Article27. Chang J, Yang JY, Choi J, Jung HH, Im GJ. Calcium imaging in gentamicin ototoxicity: increased intracellular calcium relates to oxidative stress and late apoptosis. Int J Pediatr Otorhinolaryngol. 2011; 12. 75(12):1616–1622. PMID: 22015113.
Article28. Orrenius S, McConkey DJ, Nicotera P. Role of calcium in toxic and programmed cell death. Adv Exp Med Bio. 1991; 283:419–425. PMID: 1648868.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Low Power Laser Irradiation on Gentamicin-Damaged Vestibular System in Guinea Pigs
- The Effect of Memantine on Experimentally Gentamicin Induced Vestibulotoxicity in Guinea Pigs
- Evaluation and Prevention of Gentamicin-induced Vestibulotoxicity in Rabbits Using Off-Vertical Axis Rotation
- The Protective Effect of Epigallocatechin-3-Gallate Against Gentamicin Vestibular Ototoxicity in Type I Vestibular Hair Cell of Guinea Pig
- A Promotive Effect of Low-Level Laser on Hair Cell Regeneration Following Gentamicin Induced Ototoxicity in Postnatal Organotypic Culture of Rat Utricles