Clin Exp Otorhinolaryngol.
2014 Dec;7(4):275-280. 10.3342/ceo.2014.7.4.275.
Dexmedetomidine Preconditioning Attenuates Cisplatin-Induced Ototoxicity in Zebrafish
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea. minware2@naver.com
- 2Department of Biomedical Sciences, Korea University Graduate School, Seoul, Korea.
- 3Department of Anesthesiology and Pain Medicine, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2278367
- DOI: http://doi.org/10.3342/ceo.2014.7.4.275
Abstract
OBJECTIVES
Utilisation of high-frequency drills is known to increase noise induced hearing loss due to increasing the damages of inner ear cells. This study aimed to investigate whether preconditioning by using dexmedetomidine (DEX) decreased the occurrence of ischemia in inner cells of the ear.
METHODS
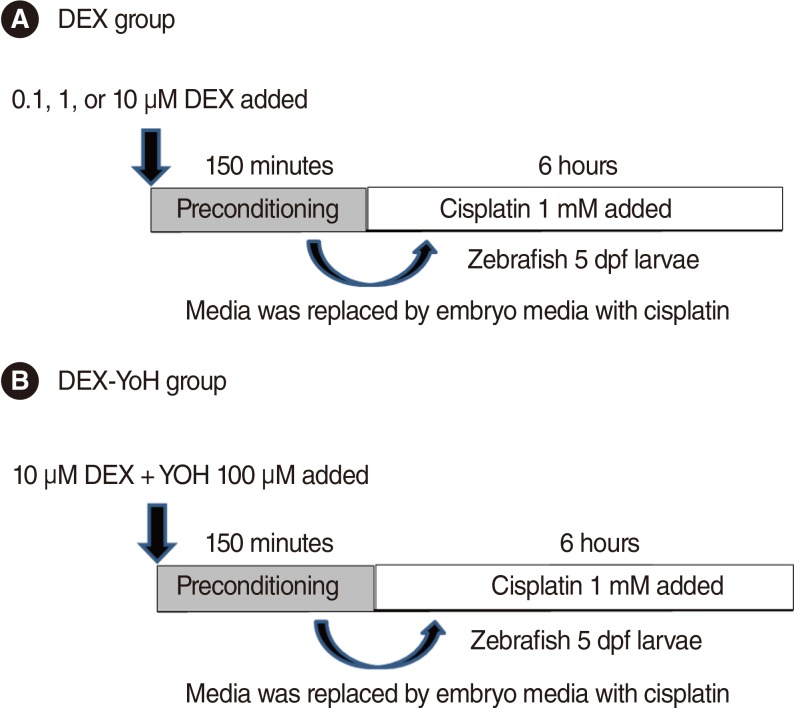
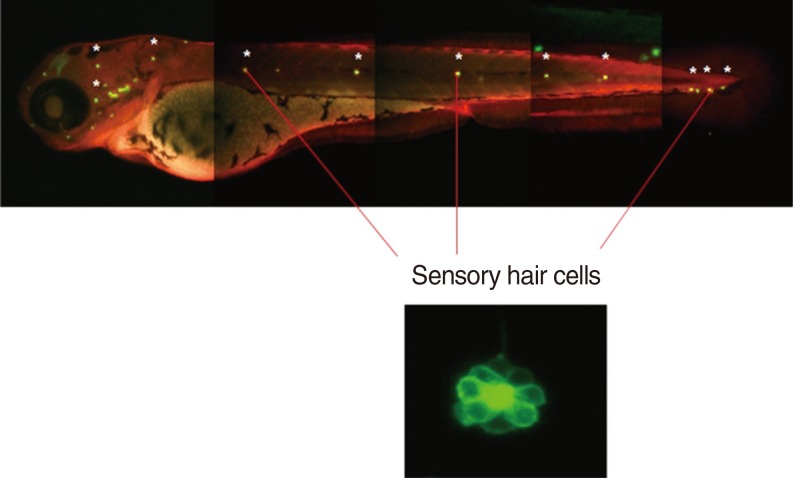
We utilised a transgenic zebrafish line Brn3C, and the embryos were collected from breeding adult zebrafish. Five-day-old larvae were cultured at the density of 50 embryos, and the larvae were classified into 4 groups: control, cisplatin group, DEX group, and DEX+yohimbine; adrenoreceptor blocker group. The DEX group was categorised into 3 subgroups by dosage; 0.1, 1, and 10 microM. Preconditioning was performed for 150 minutes and then exposed to cisplatin for 6 hours. The experiment was performed in 7 replicates for each group and the number of hair cells in 3 parts of the neuromasts of each fish was determined.
RESULTS
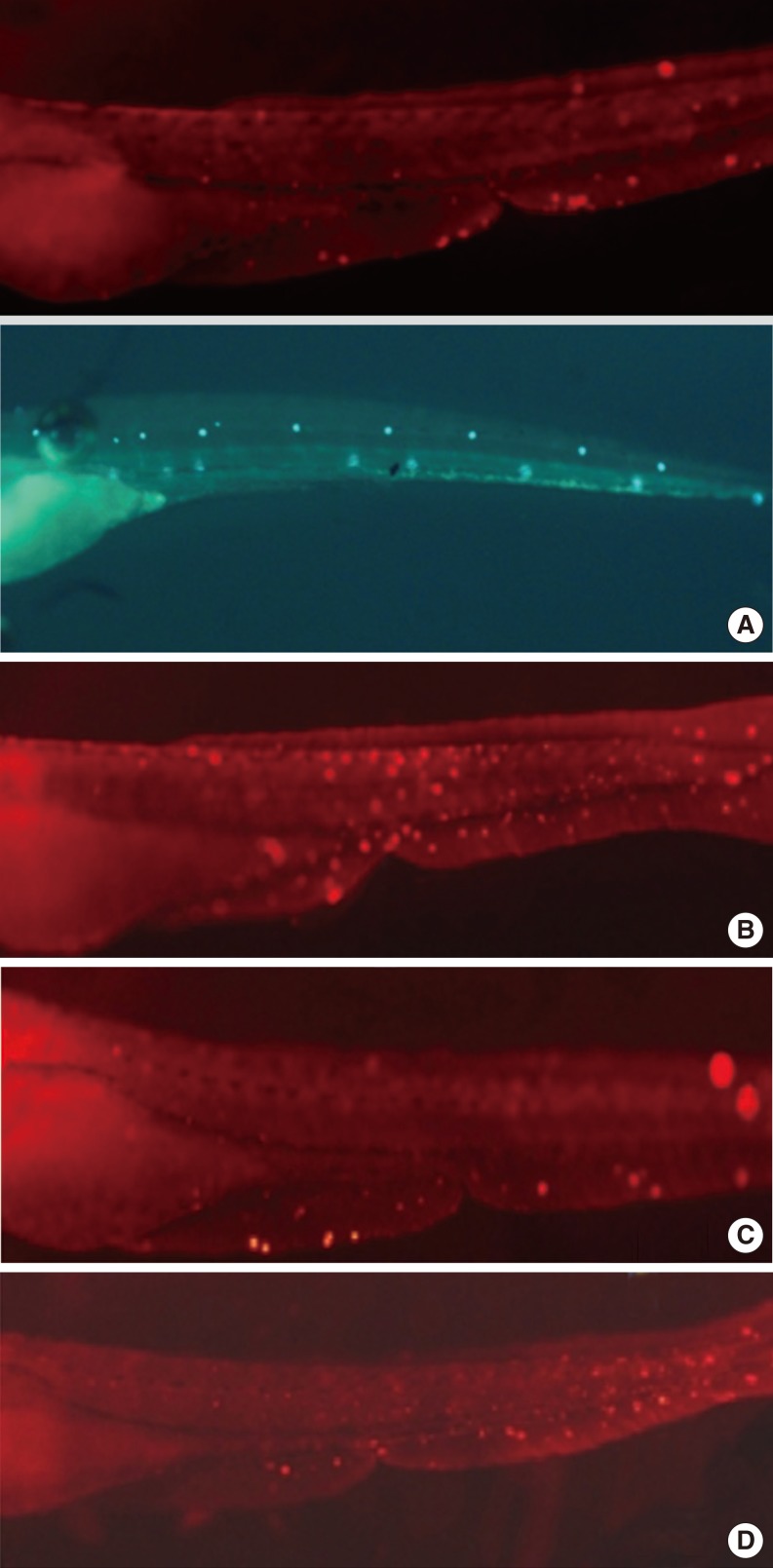
Hair cell apoptosis by cisplatin was attenuated more significantly in the DEX preconditioning group than in the control group. However, the preconditioning effects were not blocked by yohimbine.
CONCLUSION
The results of this study suggest that hearing loss caused by vibration-induced noise could be reduced by using DEX and may occur through other mechanisms rather than adreno-receptors.
Keyword
MeSH Terms
Figure
Reference
-
1. Urquhart AC, McIntosh WA, Bodenstein NP. Drill-generated sensorineural hearing loss following mastoid surgery. Laryngoscope. 1992; 6. 102(6):689–692. PMID: 1602918.
Article2. Farzanegan G, Ghasemi M, Panahi F, Raza M, Alghasi M. Does drill-induced noise have an impact on sensorineural hearing during craniotomy procedure? Br J Neurosurg. 2010; 2. 24(1):40–45. PMID: 20158351.
Article3. Hong SJ, Im GJ, Chang J, Chae SW, Lee SH, Kwon SY, et al. Protective effects of edaravone against cisplatin-induced hair cell damage in zebrafish. Int J Pediatr Otorhinolaryngol. 2013; 6. 77(6):1025–1031. PMID: 23628221.
Article4. Choi J, Im GJ, Chang J, Chae SW, Lee SH, Kwon SY, et al. Protective effects of apocynin on cisplatin-induced ototoxicity in an auditory cell line and in zebrafish. J Appl Toxicol. 2013; 2. 33(2):125–133. PMID: 22147442.
Article5. Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP, et al. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004; 10. 502(1-2):87–97. PMID: 15464093.6. Engelhard K, Werner C, Eberspacher E, Bachl M, Blobner M, Hildt E, et al. The effect of the alpha 2-agonist dexmedetomidine and the N-methyl-D-aspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003; 2. 96(2):524–531. PMID: 12538207.7. Sanders RD, Sun P, Patel S, Li M, Maze M, Ma D. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand. 2010; 7. 54(6):710–716. PMID: 20003127.
Article8. Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, et al. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009; 5. 110(5):1077–1085. PMID: 19352168.
Article9. Wang J, Meng F, Cottrell JE, Kass IS. The differential effects of volatile anesthetics on electrophysiological and biochemical changes during and recovery after hypoxia in rat hippocampal slice CA1 pyramidal cells. Neuroscience. 2006; 7. 140(3):957–967. PMID: 16580780.
Article10. Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004; 12. 101(6):1313–1324. PMID: 15564938.
Article11. Dahmani S, Paris A, Jannier V, Hein L, Rouelle D, Scholz J, et al. Dexmedetomidine increases hippocampal phosphorylated extracellular signal-regulated protein kinase 1 and 2 content by an alpha 2-adrenoceptor-independent mechanism: evidence for the involvement of imidazoline I1 receptors. Anesthesiology. 2008; 3. 108(3):457–466. PMID: 18292683.12. Steele SL, Yang X, Debiais-Thibaud M, Schwerte T, Pelster B, Ekker M, et al. In vivo and in vitro assessment of cardiac beta-adrenergic receptors in larval zebrafish (Danio rerio). J Exp Biol. 2011; 5. 214(Pt 9):1445–1457. PMID: 21490253.13. Takeda S, Hata R, Cao F, Yoshida T, Hakuba N, Hato N, et al. Ischemic tolerance in the cochlea. Neurosci Lett. 2009; 10. 462(3):263–266. PMID: 19596048.
Article14. Cason BA, Gamperl AK, Slocum RE, Hickey RF. Anesthetic-induced preconditioning: previous administration of isoflurane decreases myocardial infarct size in rabbits. Anesthesiology. 1997; 11. 87(5):1182–1190. PMID: 9366471.15. Kevin LG, Novalija E, Riess ML, Camara AK, Rhodes SS, Stowe DF. Sevoflurane exposure generates superoxide but leads to decreased superoxide during ischemia and reperfusion in isolated hearts. Anesth Analg. 2003; 4. 96(4):949–955. PMID: 12651639.
Article16. Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000; 5. 28(10):1456–1462. PMID: 10927169.
Article17. Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, et al. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol. 2007; 5. 292(5):C1583–C1590. PMID: 17215328.
Article18. Rybak LP, Kelly T. Ototoxicity: bioprotective mechanisms. Curr Opin Otolaryngol Head Neck Surg. 2003; 10. 11(5):328–333. PMID: 14502062.
Article19. Rybak LP. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg. 2007; 10. 15(5):364–369. PMID: 17823555.
Article20. Kovacic P, Somanathan R. Ototoxicity and noise trauma: electron transfer, reactive oxygen species, cell signaling, electrical effects, and protection by antioxidants: practical medical aspects. Med Hypotheses. 2008; 70(5):914–923. PMID: 17977665.
Article21. Ruuskanen JO, Peitsaro N, Kaslin JV, Panula P, Scheinin M. Expression and function of alpha-adrenoceptors in zebrafish: drug effects, mRNA and receptor distributions. J Neurochem. 2005; 9. 94(6):1559–1569. PMID: 16000146.22. Chang Y, Huang X, Liu Z, Han G, Huang L, Xiong YC, et al. Dexmedetomidine inhibits the secretion of high mobility group box 1 from lipopolysaccharide-activated macrophages in vitro. J Surg Res. 2013; 5. 181(2):308–314. PMID: 22939552.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphologic Study of Effect of Fosfomycin and Pentoxifylline on Cisplatin Induced Ototoxicity in Guinea Pig
- Cisplatin-Induced Ototoxicity: Updates on Potential Molecular Mechanism
- Protective Effect of Procaine Hydrochloride on Cisplatin Induced Ototoxicity in Guinea Pig
- Preventive Effect of Fosfomycin on Cisplatin Induced Ototoxicity in Cochlea of Guinea Pig
- Nurses’ and Medical Doctors’ Perception and Management Status on Ototoxicity Related to Chemotherapy