Korean J Pain.
2014 Jul;27(3):219-228. 10.3344/kjp.2014.27.3.219.
Therapeutic Effect of Epidurally Administered Lipo-Prostaglandin E1 Agonist in a Rat Spinal Stenosis Model
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Jeju National University Hospital, Jeju, Korea.
- 2Department of Anesthesiology and Pain Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. painfree@snubh.org
- 3Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Anesthesiology and Pain Medicine, Seoul National University Hospital, Seoul, Korea.
- 5Department of Anesthesiology and Pain Medicine, Seoul National University School of Medicine, Seoul, Korea.
- KMID: 2278226
- DOI: http://doi.org/10.3344/kjp.2014.27.3.219
Abstract
- BACKGROUND
A lipo-prostaglandin E1 agonist is effective for the treatment of neurological symptoms of spinal stenosis when administered by an oral or intravenous route. we would like to reveal the therapeutic effect of an epidural injection of lipo-prostaglandin E1 on hyperalgesia in foraminal stenosis.
METHODS
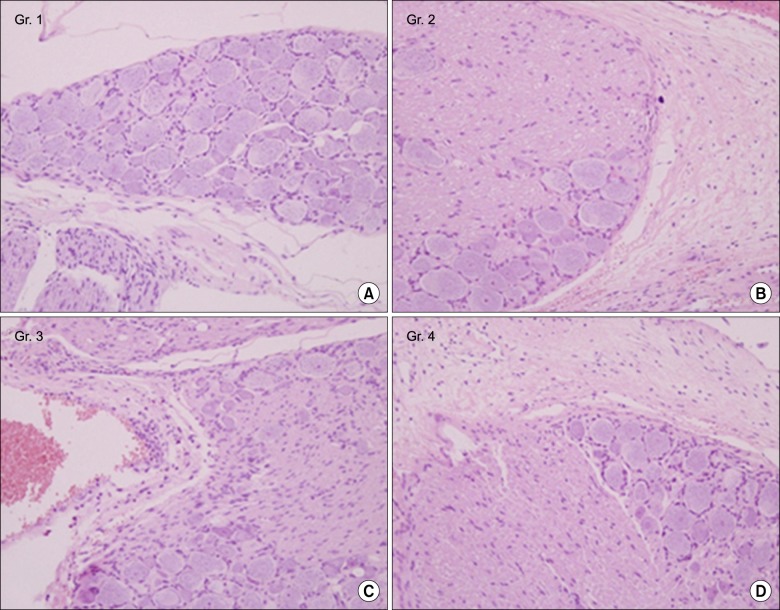
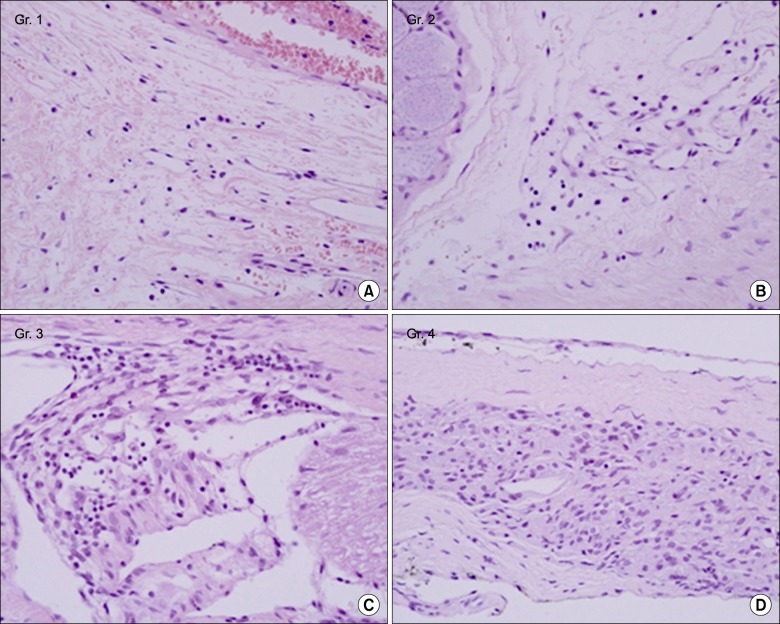
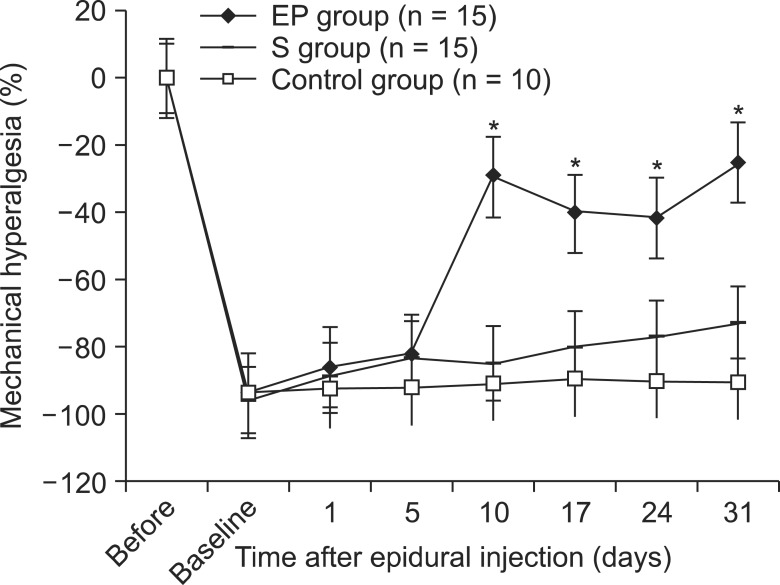
A total of 40 male Sprague-Dawley rats were included. A small stainless steel rod was inserted into the L5/L6 intervertebral foramen to produce intervertebral foraminal stenosis and chronic compression of the dorsal root ganglia (DRG). The rats were divided into three groups: epidural PGE1 (EP) (n = 15), saline (n = 15), and control (n = 10). In the EP group, 0.15 microg.kg-1 of a lipo-PGE1 agonist was injected daily via an epidural catheter for 10 days from postoperative day 3. In the saline group, saline was injected. Behavioral tests for mechanical hyperalgesia were performed for 3 weeks. Then, the target DRG was analyzed for the degree of chromatolysis, chronic inflammation, and fibrosis in light microscopic images.
RESULTS
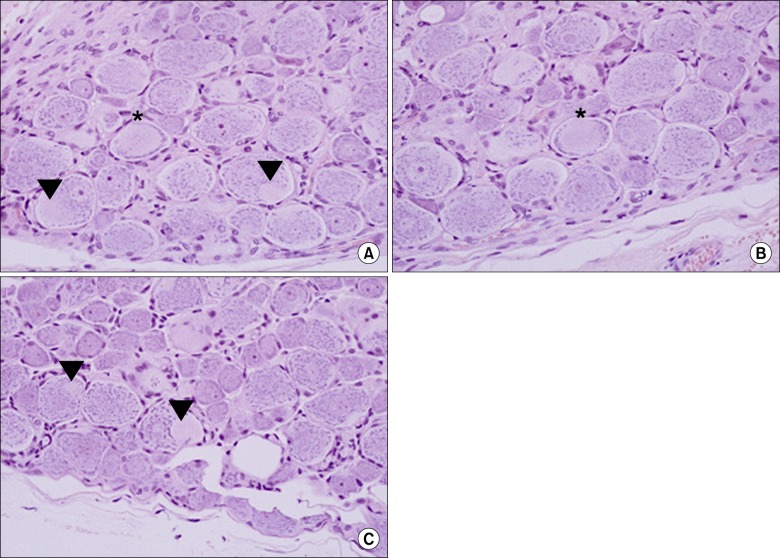
From the fifth day after lipo-PGE1 agonist injection, the EP group showed significant recovery from mechanical hyperalgesia, which was maintained for 3 weeks (P < 0.05). Microscopic analysis showed much less chromatolysis in the EP group than in the saline or control groups.
CONCLUSIONS
An epidurally administered lipo-PGE1 agonist relieved neuropathic pain, such as mechanical hyperalgesia, in a rat foraminal stenosis model, with decreasing chromatolysis in target DRG. We suggest that epidurally administered lipo-PGE1 may be a useful therapeutic candidate for patients with spinal stenosis.
MeSH Terms
Figure
Cited by 1 articles
-
Effect of epidural polydeoxyribonucleotide in a rat model of lumbar foraminal stenosis
Ho-Jin Lee, Jiyoun Ju, Eunjoo Choi, Francis Sahngun Nahm, Ghee Young Choe, Pyung Bok Lee
Korean J Pain. 2021;34(4):394-404. doi: 10.3344/kjp.2021.34.4.394.
Reference
-
1. Goupille P, Jayson MI, Valat JP, Freemont AJ. The role of inflammation in disk herniation-associated radiculopathy. Semin Arthritis Rheum. 1998; 28:60–71. PMID: 9726337.
Article2. Thomas SA. Spinal stenosis: history and physical examination. Phys Med Rehabil Clin N Am. 2003; 14:29–39. PMID: 12622480.
Article3. Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998; 77:15–23. PMID: 9755014.
Article4. Cavanaugh JM, Ozaktay AC, Yamashita T, Avramov A, Getchell TV, King AI. Mechanisms of low back pain: a neurophysiologic and neuroanatomic study. Clin Orthop Relat Res. 1997; (335):166–180. PMID: 9020216.
Article5. Akuthota V, Lento P, Sowa G. Pathogenesis of lumbar spinal stenosis pain: why does an asymptomatic stenotic patient flare? Phys Med Rehabil Clin N Am. 2003; 14:17–28. PMID: 12622479.
Article6. Mizushima Y, Yanagawa A, Hoshi K. Prostaglandin E1 is more effective, when incorporated in lipid microspheres, for treatment of peripheral vascular diseases in man. J Pharm Pharmacol. 1983; 35:666–667. PMID: 6139437.
Article8. Murakami M, Takahashi K, Sekikawa T, Yasuhara K, Yamagata M, Moriya H. Effects of intravenous lipoprostaglandin E1 on neurogenic intermittent claudication. J Spinal Disord. 1997; 10:499–504. PMID: 9438815.
Article9. Yone K, Sakou T, Kawauchi Y. The effect of Lipo prostaglandin E1 on cauda equina blood flow in patients with lumbar spinal canal stenosis: myeloscopic observation. Spinal Cord. 1999; 37:269–274. PMID: 10338347.
Article10. Matsudaira K, Seichi A, Kunogi J, Yamazaki T, Kobayashi A, Anamizu Y, et al. The efficacy of prostaglandin E1 derivative in patients with lumbar spinal stenosis. Spine (Phila Pa 1976). 2009; 34:115–120. PMID: 19112336.
Article11. Mizushima Y. Lipo-prostaglandin preparations. Prostaglandins Leukot Essent Fatty Acids. 1991; 42:1–6. PMID: 2011606.
Article12. Sekikawa T, Murakami M, Takahashi K, Yamagata M, Yasuhara K, Nemoto T, et al. Effects of lipo-prostaglandin E1 on blood flow and oxygen pressure in lumbo-sacral nerve roots. J Orthop Sci. 1997; 2:289–294.
Article13. Kawamura T, Akira T, Watanabe M, Kagitani Y. Prostaglandin E1 prevents apoptotic cell death in superficial dorsal horn of rat spinal cord. Neuropharmacology. 1997; 36:1023–1030. PMID: 9294966.
Article14. Kawamura T, Horie S, Maruyama T, Akira T, Imagawa T, Nakamura N. Prostaglandin E1 transported into cells blocks the apoptotic signals induced by nerve growth factor deprivation. J Neurochem. 1999; 72:1907–1914. PMID: 10217267.
Article15. Gensch C, Clever Y, Werner C, Hanhoun M, Böhm M, Laufs U. Regulation of endothelial progenitor cells by prostaglandin E1 via inhibition of apoptosis. J Mol Cell Cardiol. 2007; 42:670–677. PMID: 17291526.
Article16. Lim YJ, Sim WS, Kim YC, Lee SC, Choi YL. The neurotoxicity of epidural hyaluronic acid in rabbits: a light and electron microscopic examination. Anesth Analg. 2003; 97:1716–1720. PMID: 14633548.
Article17. Kim YC, Lim YJ, Lee SC. Spreading pattern of epidurally administered contrast medium in rabbits. Acta Anaesthesiol Scand. 1998; 42:1092–1095. PMID: 9809094.
Article18. Kawakami M, Weinstein JN, Spratt KF, Chatani K, Traub RJ, Meller ST, et al. Experimental lumbar radiculopathy. Immunohistochemical and quantitative demonstrations of pain induced by lumbar nerve root irritation of the rat. Spine (Phila Pa 1976). 1994; 19:1780–1794. PMID: 7526474.
Article19. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63. PMID: 7990513.
Article20. Dixon WJ, Massey FJ. Introduction to statistical analysis. 3rd ed. New York (NY): McGraw-Hill;1969.21. Safonova GD, Kovalenko AP. Morphofunctional characteristics of neurons in the spinal ganglia of the dog in the post-distraction period. Neurosci Behav Physiol. 2006; 36:491–494. PMID: 16645763.
Article22. Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989; 73:383–389. PMID: 2915862.
Article23. Rydevik BL, Myers RR, Powell HC. Pressure increase in the dorsal root ganglion following mechanical compression. Closed compartment syndrome in nerve roots. Spine (Phila Pa 1976). 1989; 14:574–576. PMID: 2749371.
Article24. Andreollo NA, Santos EF, Araújo MR, Lopes LR. Rat's age versus human's age: what is the relationship? Arq Bras Cir Dig. 2012; 25:49–51. PMID: 22569979.25. Nakai K, Takenobu Y, Takimizu H, Akimaru S, Ito H, Maegawa H, et al. Effects of orally administered OP-1206 alpha-CD with loxoprofen-Na on walking dysfunction in the rat neuropathic intermittent claudication model. Prostaglandins Leukot Essent Fatty Acids. 2003; 69:269–273. PMID: 12907137.
Article26. Shirasaka M, Takayama B, Sekiguchi M, Konno S, Kikuchi S. Vasodilative effects of prostaglandin E1 derivate on arteries of nerve roots in a canine model of a chronically compressed cauda equina. BMC Musculoskelet Disord. 2008; 9:41. PMID: 18394203.
Article27. Yoon HK, Lee PB, Han JS, Park SH, Lee SY, Kim YH, et al. The effect of intravenous lipo-prostaglandin E1 injectioin in a rat foraminal stenosis model. Korean J Pain. 2007; 20:15–20.
Article28. Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol. 1999; 82:3347–3358. PMID: 10601466.
Article29. Epstein NE. The risks of epidural and transforaminal steroid injections in the Spine: commentary and a comprehensive review of the literature. Surg Neurol Int. 2013; 4:S74–S93. PMID: 23646278.
Article30. Hirano A. Neurons and astrocytes. In : Davis RL, Robertson DM, editors. Textbook of neuropathology. 3rd ed. Baltimore (MA): Williams & Wilkins;1997. p. 5–7.31. Levine S, Saltzman A, Kumar AR. A method for peripheral chromatolysis in neurons of trigeminal and dorsal root ganglia, produced in rats by lithium. J Neurosci Methods. 2004; 132:1–7. PMID: 14687669.
Article32. Naruo S, Okajima K, Taoka Y, Uchiba M, Nakamura T, Okabe H, et al. Prostaglandin E1 reduces compression trauma-induced spinal cord injury in rats mainly by inhibiting neutrophil activation. J Neurotrauma. 2003; 20:221–228. PMID: 12675974.
Article33. Manchikanti L, Boswell MV, Datta S, Fellows B, Abdi S, Singh V, et al. Comprehensive review of therapeutic interventions in managing chronic spinal pain. Pain Physician. 2009; 12:E123–E198. PMID: 19668281.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Intravenous Lipo-Prostaglandin E1 Injectioin in a Rat Foraminal Stenosis Model
- Therapeutic Effects of Lipo-Prostaglandin E1 in Buerger's Disease
- Therapeutic Effects of Carbogen Inhalation and Lipo-Prostaglandin E1 in Sudden Hearing Loss
- The Effects of Lipo Prostaglandin E1(Eglandin ) in Patients with Ductus Dependent Congenital Heart Disease
- Analysis of Therapeutic Effects of Lipo-Prostaglandin E1 for Treatment of Sudden Sensorineural Hearing Loss in Patients with Type 2 Diabetes